Accordion pill comprising levodopa for an improved treatment of parkinson's disease symptoms
a technology of accordion and levodopa, which is applied in the direction of biocide, drug composition, peptide/protein ingredients, etc., can solve the problems of affecting the movement of patients, the action of each dose tends to wear off in the majority of patients with pd, and the inability to move easily, etc., to improve sleep quality, alleviate or eliminate the symptoms of morning akinesia or morning dystonia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
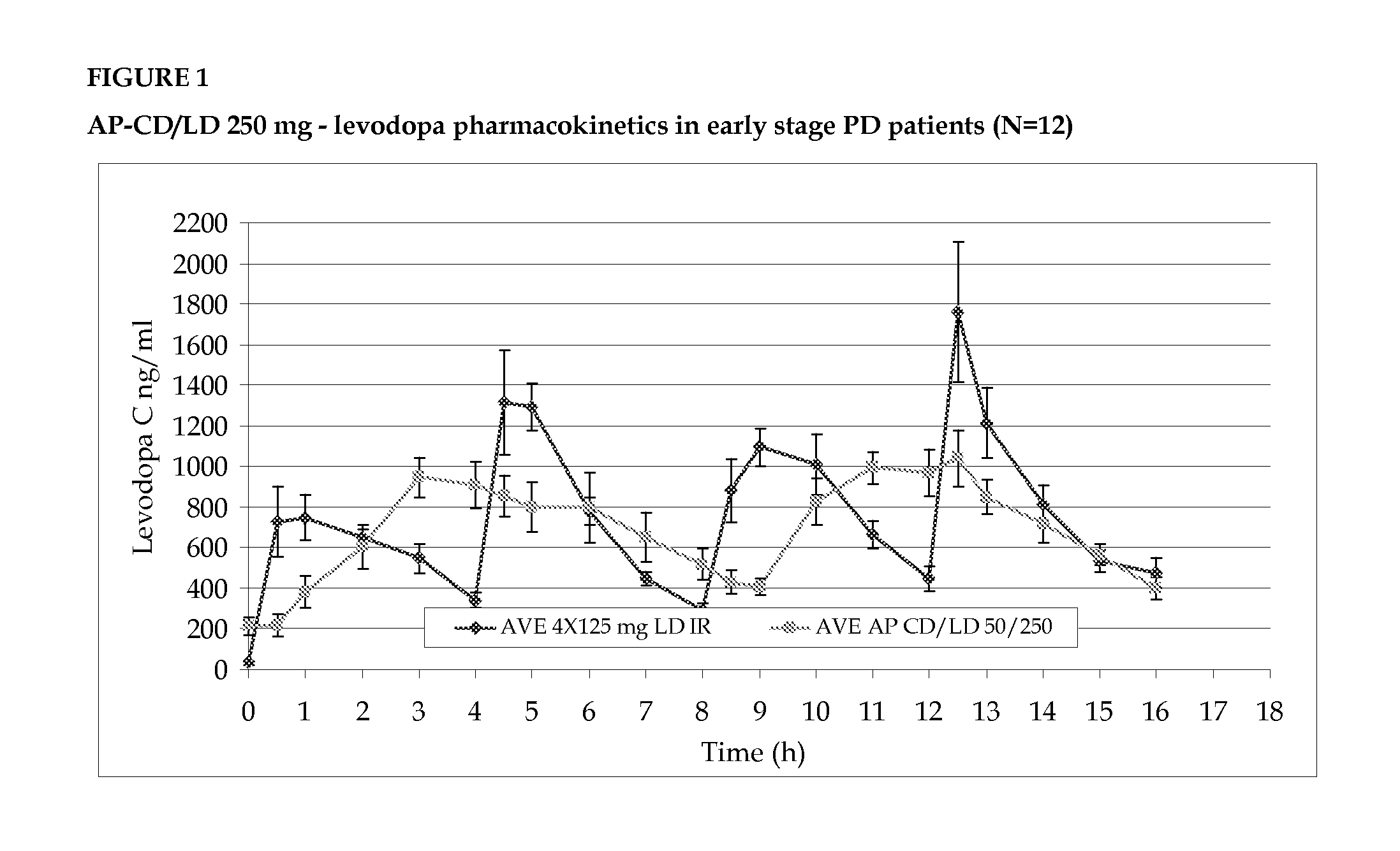
Phase IIA Included 12 Early Stage PD Patients
Clinical Study Design:
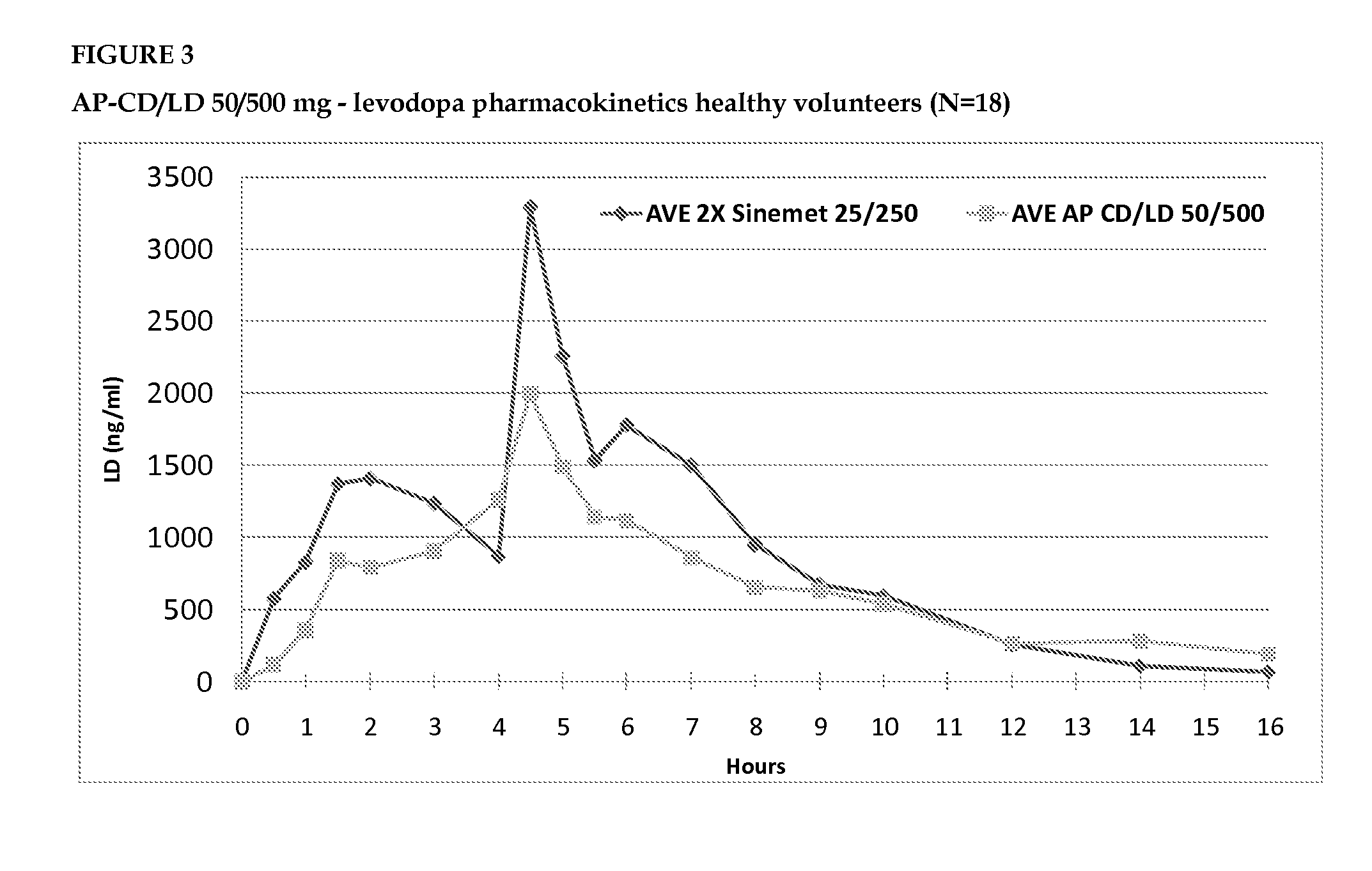
[0096]A multi center, open, two-way randomized crossover, multiple dose, active control, pharmacokinetic study in Parkinson's patients that are not experiencing wearing off, treated with low dose AP-CD / LD
[0097]The group was crossed over with a daily equidose of immediate release (IR) preparations of carbidopa / levodopa.
The Objectives
[0098]The primary objective was to evaluate the blood level profile of the AP-CD / LD relative to that of IR carbidopa / levodopa.
[0099]Another objective was to monitor the subjects for adverse events during the study period and to compare the safety of the test products with the reference products.
The Course of the Study
[0100]Subjects were randomized to start with either AP-CD / LD or with IR-CD / LD. The AP-CD / LD was dosed at 0 and 8 hrs on each day, for seven days. The reference product was the commercially available 25 / 250 mg Dopicar® (Teva Pharmaceuticals) CD / LD immediate-release tablet (IR-C...
example 2
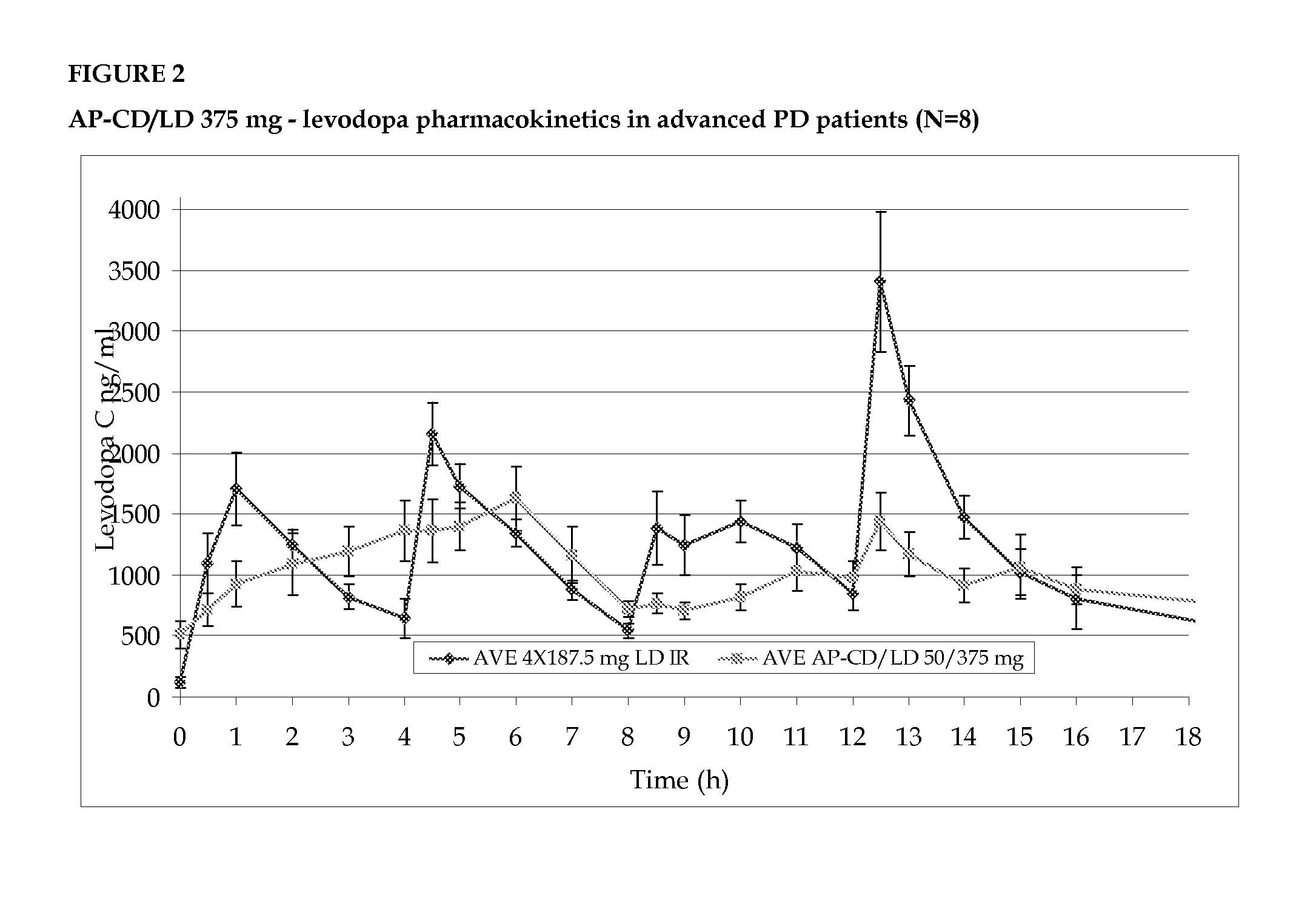
Phase IIB—12 Fluctuating PD Patients
[0110]The purpose of this study was to evaluate the efficacy (pharmacokinetics and pharmacodynamics) and the safety of AP-CD / LD 50 / 375 mg, in various groups of advanced PD patients, after multiple dosing, in comparison to CD / LD formulations, currently on the market.
Clinical Study Design
[0111]A multi center, open, two-way randomized crossover, multiple dose, active control, pharmacokinetic and pharmacodynamic study in patients with wearing off treated with high dose AP-CD / LD.
[0112]The group was crossed over with the patient's current treatment dose. The study was conducted in three medical centers.
The Objectives:
[0113]The primary objectives of the study was to evaluate the pharmacokinetic profile of AP-CD / LD relative to that of IR-CD / LD and to determine the relative pharmacodynamic profiles of the AP-CD / LD vs. IR-CD / LD under real conditions of use (i.e. derived from at-home diary entries). Another objective was to monitor the subjects for adverse e...
example 3
Pharmacodynamic Evaluation in Fluctuating Patients
[0151]The purpose of this study was to evaluate pharmacodynamic changes in fluctuating PD patients upon treatment with AP-CD / LD 50 / 375 mg, following three weeks treatment.
Study Objectives:
[0152]Primary objectives of the study were to evaluate a change in the total daily OFF time (hr) from at home ON / OFF diaries, at week 3 of each treatment, between AP-CD / LD and active control; and to assess patient and investigator global evaluation, and degree of satisfaction with, AP-CD / LD relative to current levodopa treatment.
Course of the Study:
[0153]The study included multiple dosing for 21 days with the AP-CD / LD crossed over with a similar duration of treatment with the patient's current therapy. Both treatment periods included 21 days of treatment out of which the first 14 days were for equilibration (readjusting to the treatment after the crossover) and the last 4 days were for the evaluation (test period).
[0154]Subjects were randomized to s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total OFF time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com