Isosorbide mononitrate micro-porous osmotic pump tablet and preparation method thereof
A technology of isosorbide dinitrate and osmotic pump tablets, applied in the directions of pharmaceutical formulation, drug delivery, sugar-coated pills, etc., can solve the problems such as unavoidable complex process, unstable in vivo effect, unavoidable trouble, etc. The effect of stable blood concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0029] Chip composition:
[0030]
[0031] Coating liquid composition:
[0032]
[0033] Preparation process: the raw and auxiliary materials are respectively passed through a 100-mesh sieve, and the isosorbide mononitrate, the osmotic pressure active substance and the filler are mixed through a 60-mesh sieve and mixed evenly, and an appropriate amount of 5% polyvinylpyrrolidone K30 ethanol solution is added to make a soft material, and a 20-mesh sieve is used Granulate, dry at 40°C, sieve through a 18-mesh sieve, add talcum powder and press into tablet cores. Dissolve cellulose acetate, plasticizer and porogen in acetone, coat the tablet core, increase the weight to the desired thickness, and cure at 40°C for 12 hours.
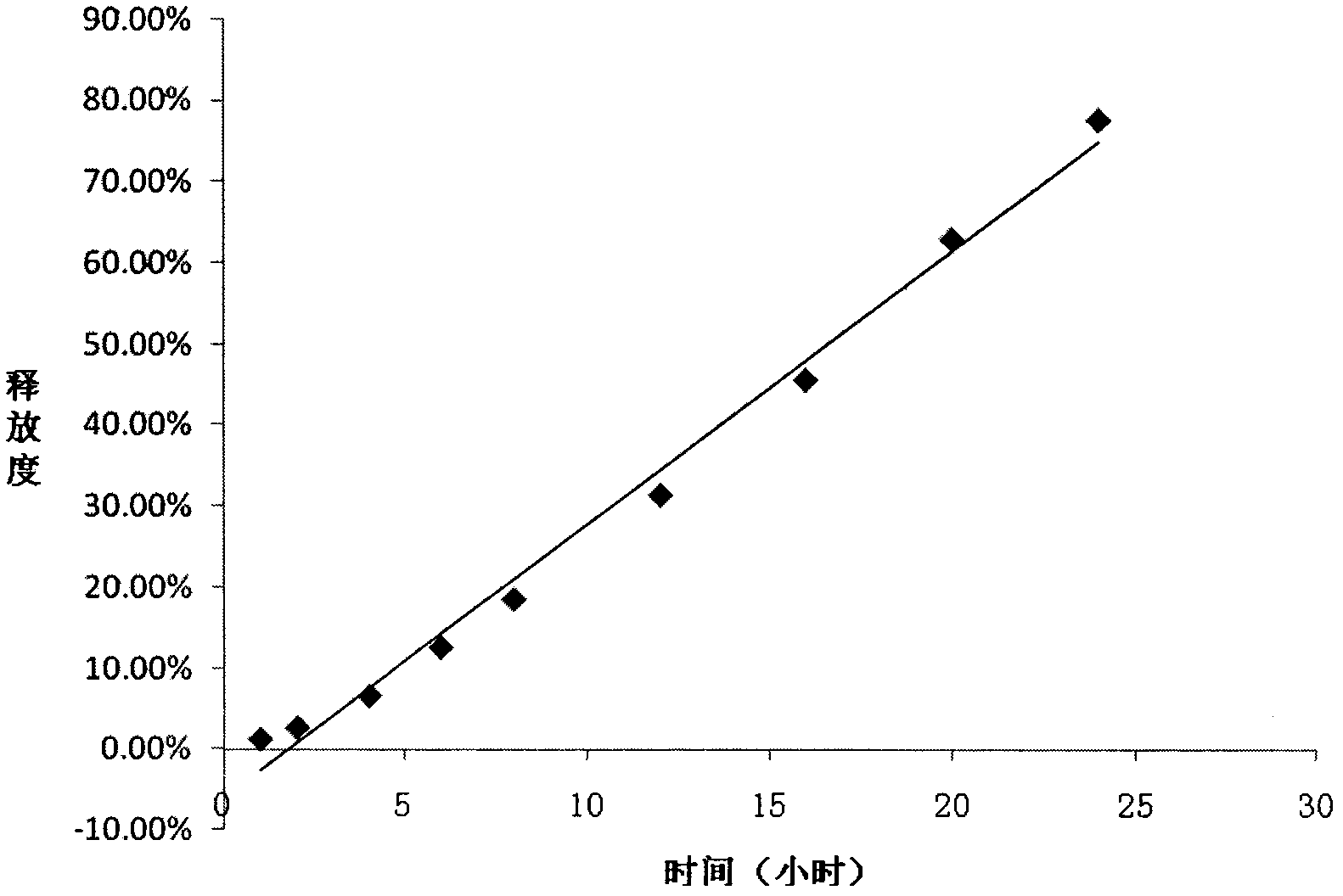
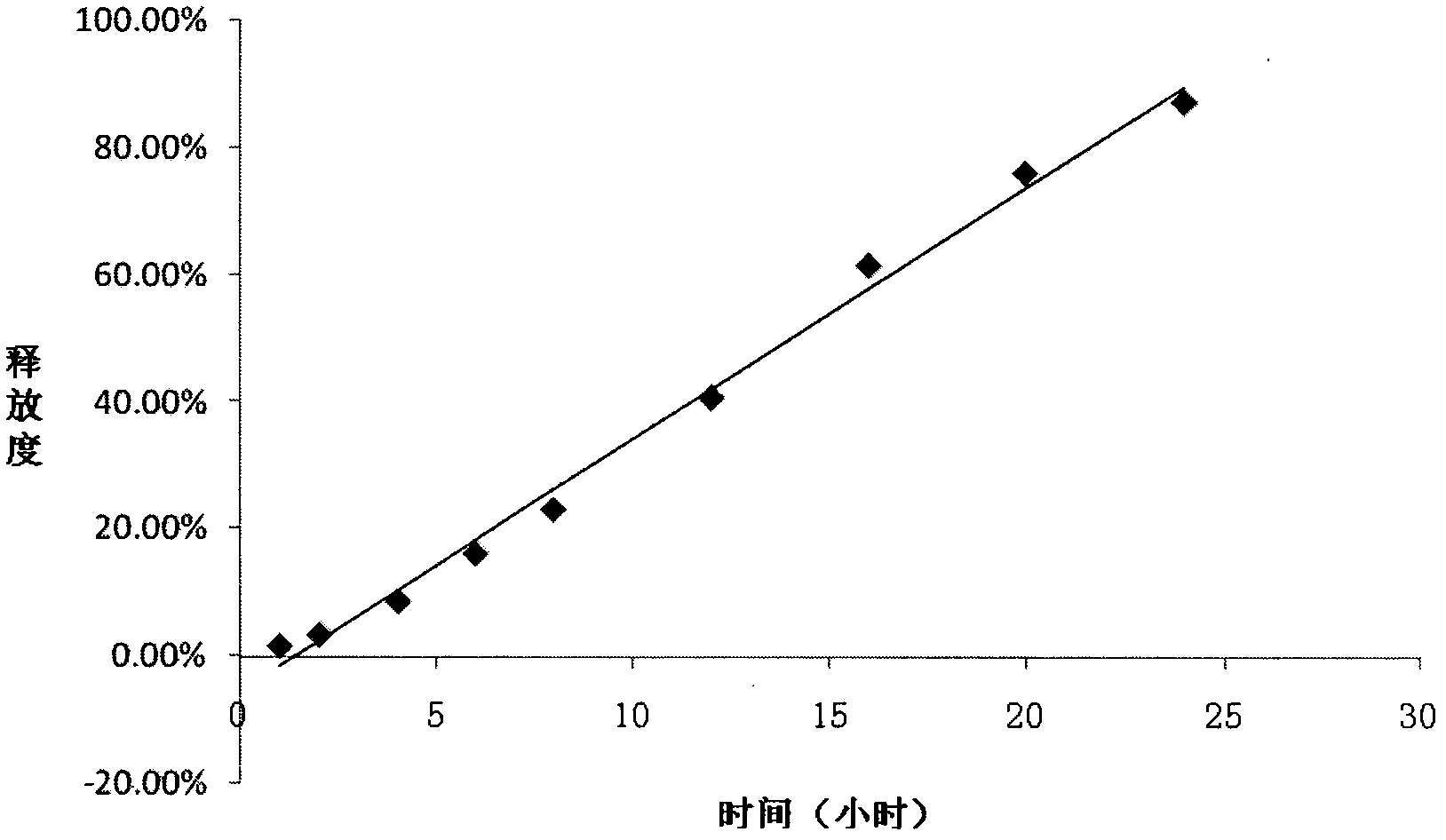
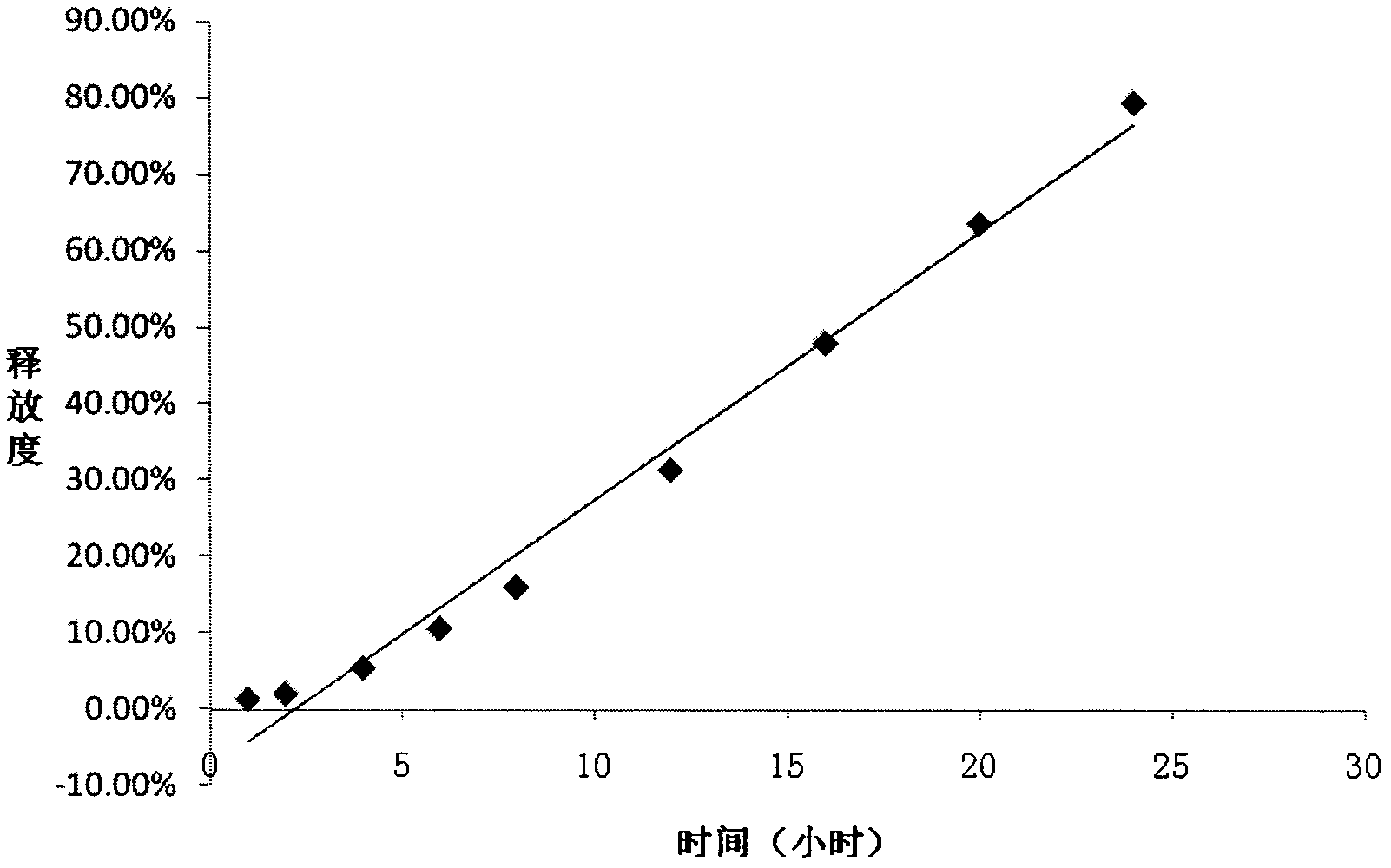
[0034] Example 2 with the best effect was selected to evaluate the release characteristics of the drug in release media with different pH and different osmotic pressures.
[0035] The present invention has selected four kinds of release media (pH1, pH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com