Pharmaceutical compositions having a selected release duration
A composition and polymer technology, applied in the direction of drug delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as not suitable for patients, and achieve the effect of easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
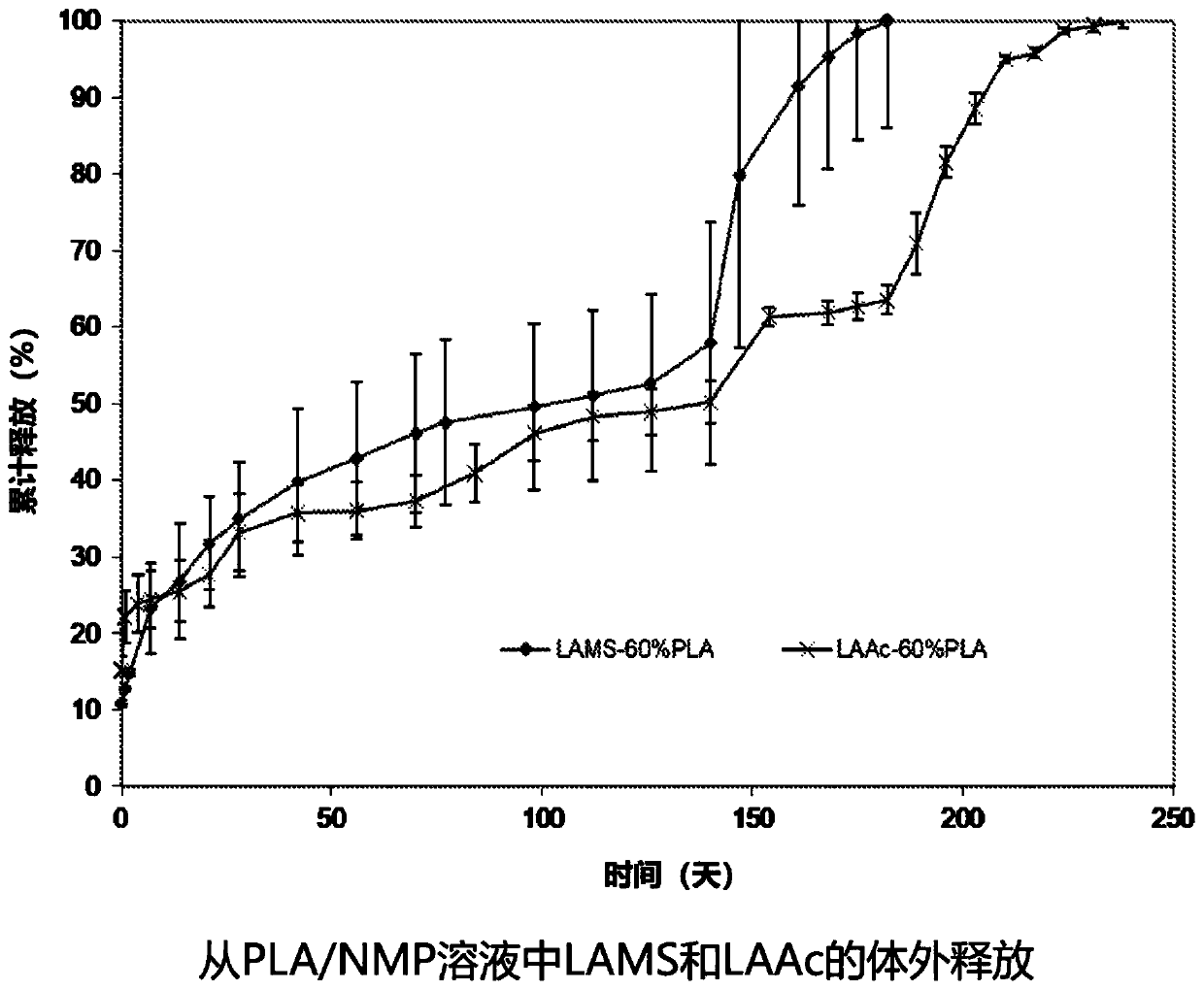
[0116] Example 1: Stability of peptide agents and biodegradable polymers in injectable polymer compositions
[0117] Poly(DL-lactide-co-glycolide) (PLGA) having a lactide to glycolide ratio of 85 / 15 with laurate end groups was dissolved in N-methyl-2-pyrrolidone ( NMP) to obtain a 50% by weight solution. The leuprolide salt and PLGA solution were mixed in NMP at the ratios shown in Table 1 to obtain a homogeneous injectable composition. The injectable composition is filled into a 1.2 mL polypropylene syringe with a luer-lock tip. The prefilled syringe is then sealed using the Luer lock cap. Capped syringes were packaged in containers and sealed under vacuum in plastic bags, then stored at 4°C and room temperature (approximately 22°C) for up to 18 months. Injectable compositions were sampled at 24 hour, 1, 2, 3, 6, 12 and 18 month time points. The purity of leuprolide in the samples was determined by HPLC. The molecular weight of the polymers was determined by gel permeati...
Embodiment 2
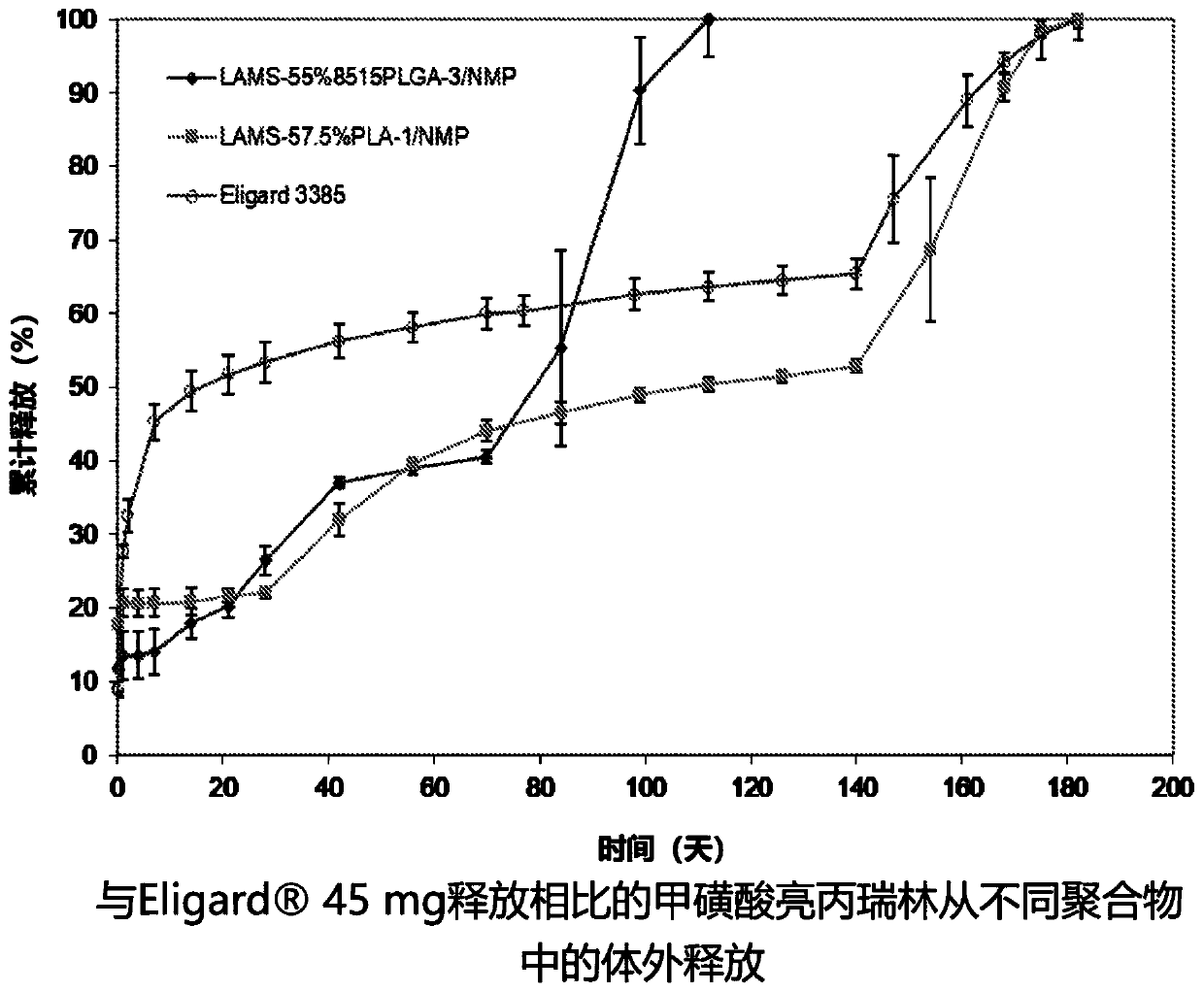
[0130] Example 2: Delivery of Leuprolide Over 6 Months
[0131] 45 mg provides continuous delivery of leuprolide acetate for 6 months. The product is supplied in two separate syringes, leuprolide acetate in one syringe and polymer solution in the other. Mix the two immediately before injection. The polymer solution contained a 50% solution of 8515PLGA polymer in NMP. The molecular weight of the polymer is about 20k Daltons. Comparable formulations were made using leuprolide mesylate at a molar ratio of methanesulfonic acid to leuprolide of 1.55:1 (LAMS(1.55)) and a similar 8515PLGA polymer. The polymer also has a molecular weight of approximately 20,000 Daltons and a polydispersity index (PDI) of 1.7. Leuprolide mesylate is prepared from leuprolide acetate by ion-exchange lyophilization, which contains a small amount of acetate. The formulations are mixed and stored in a single syringe. Another formulation was prepared with LAMS using a PLA polymer with a molecular wei...
Embodiment 3
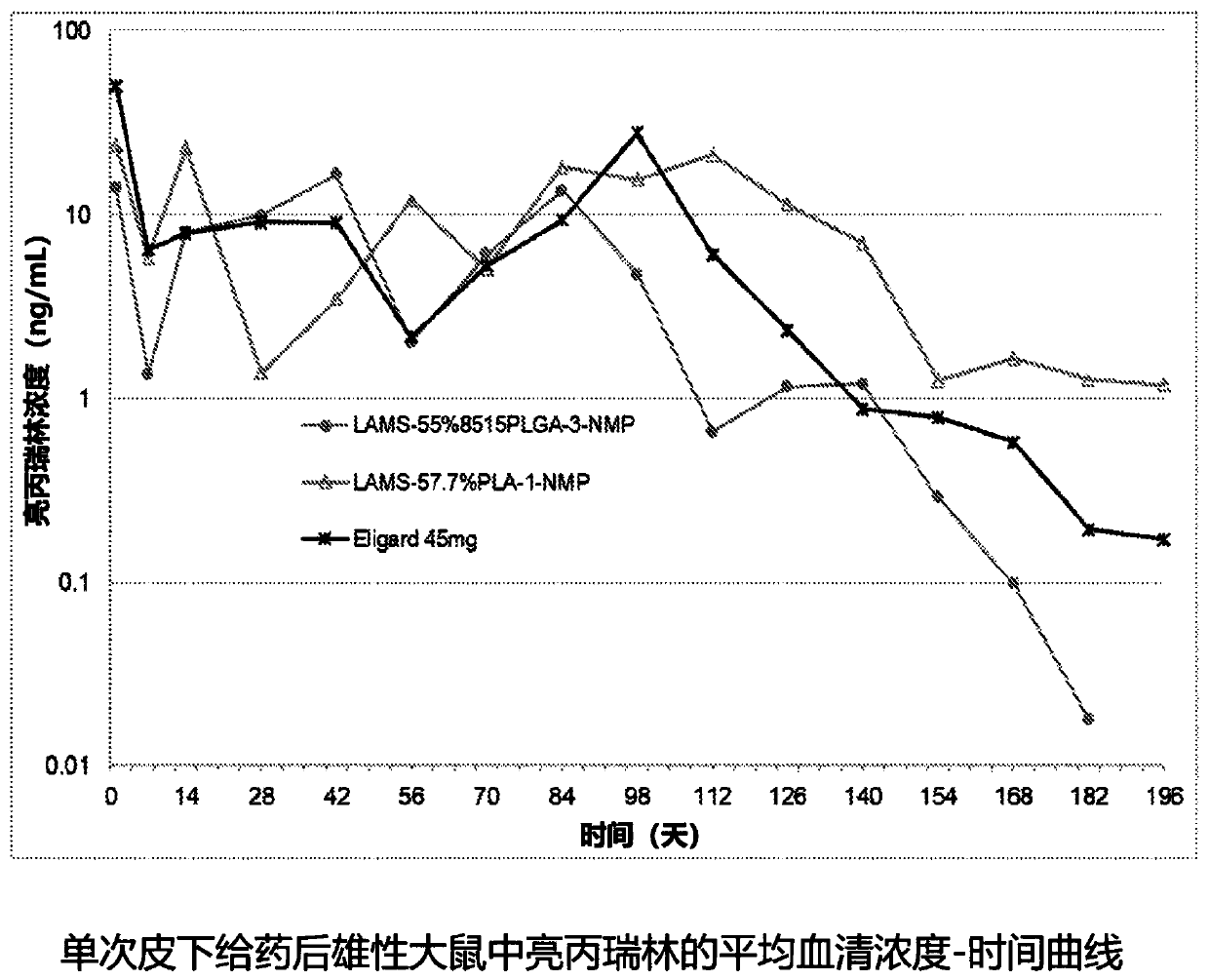
[0133] Example 3: In vivo delivery of leuprolide over 6 months
[0134] The aim of this study was to characterize the pharmacokinetics of a depot containing leuprolide mesylate after a single subcutaneous administration in male rats for 7 months and to investigate the effect of different polymers following SC administration and activity of the preparation. 45 mg was used as a reference drug. Leuprolide (LA) release was determined by analyzing the serum concentration of LA over time following SC administration.
[0135] Male rats were divided into groups (6 per group) and received the following leuprolide mesylate (LAMS 1.55) formulations. 55.2% 8515PLGA-3 / 44.8% NMP (irradiation dose 25kGy), 57.6% PLA-1 / 42.4% NMP (irradiation dose 25kGy), or reference drug 45mg. 8515PLGA-3 polymer has a molecular weight similar to 45 mg (20k) molecular weight, the PLA-1 polymer has a molecular weight of 15k. Blood samples were collected before and after dosing up to Day 196. figure 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com