Patents
Literature
41 results about "Airways disease" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Implant for treatment of sleep disorders
InactiveUS20070144535A1Reduce and eliminate rockingMinimal tissue damageSnoring preventionNon-surgical orthopedic devicesDiseaseTreatment sleep
An airway implant device for maintaining and / or creating an opening in air passageways is disclosed. Methods of using the device are also disclosed. The airway implant device comprises an actuator element to control the opening of an air passageway. Preferably the actuator element is an electroactive polymer element. Energizing of the electroactive polymer element provides support for the walls in order to prevent the collapse of an air passageway. Some embodiments of the invention include a sensor capable of sensing the possible occurrence of an apneic event and activating the actuator element of the airway implant device. Some embodiments include a housing designed to conform to the shape of the palate. Some embodiments include an attachment element to secure the device to tissue. Methods of treating airway disorders such as sleep apnea and snoring with the airway implant device are disclosed herein.
Owner:PAVAD MEDICAL
Tetradydro-Naphthalene And Urea Derivatives
This invention relates to tetrahydro-naphthalene and urea derivatives and salts thereof which are useful as active ingredients of pharmaceutical preparations. The tetrahydro-naphthalene and urea derivatives of the present invention have vanilloid receptor (VR1) antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with VR1 activity, in particular for the treatment of urological diseases or disorders, such as detrusor overactivity (overactive bladder), urinary incontinence, neurogenic detrusor overactivity (detrusor hyperflexia), idiopathic detrusor overactivity (detrusor instability), benign prostatic hyperplasia, and lower urinary tract symptoms; chronic pain, neuropathic pain, postoperative pain, rheumatoid arthritic pain, neuralgia, neuropathies, algesia, nerve injury, ischaemia, neurodegeneration, stroke, and inflammatory disorders such as asthma and chronic obstructive pulmonary (or airways) disease (COPD).
Owner:BAYER SCHERING PHARMA AG
Compositions comprising an antimuscarinic and a long-acting beta-agonist
InactiveUS20090181935A1Substantial therapeutic benefitQuick effectBiocideAnimal repellantsDermatologyAirways disease
Compositions which comprise a combination of a salt of 3-[[[(3-fluorophenyl)[(3,4,5-trifluoro phenyl)methyl]amino]carbonyl]oxy]-1-[2-oxo-2-(2-thienyl)ethyl]-1-azoniabicyclo [2.2.2]octane, and a long-acting phenylalkylamino beta2-agonist are effective for the prevention and treatment of inflammatory or obstructive airways diseases.
Owner:CHIESI FARM SPA
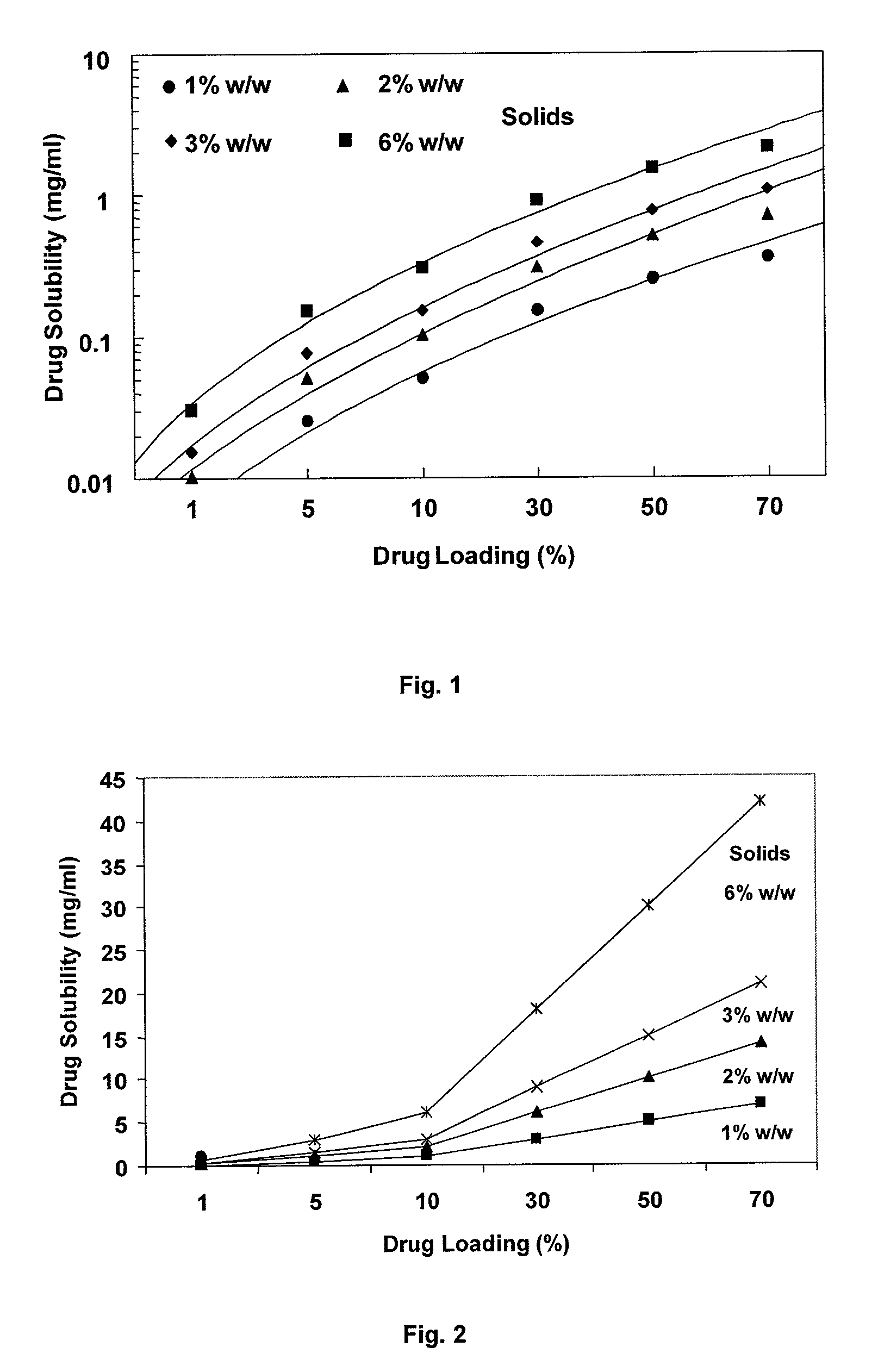
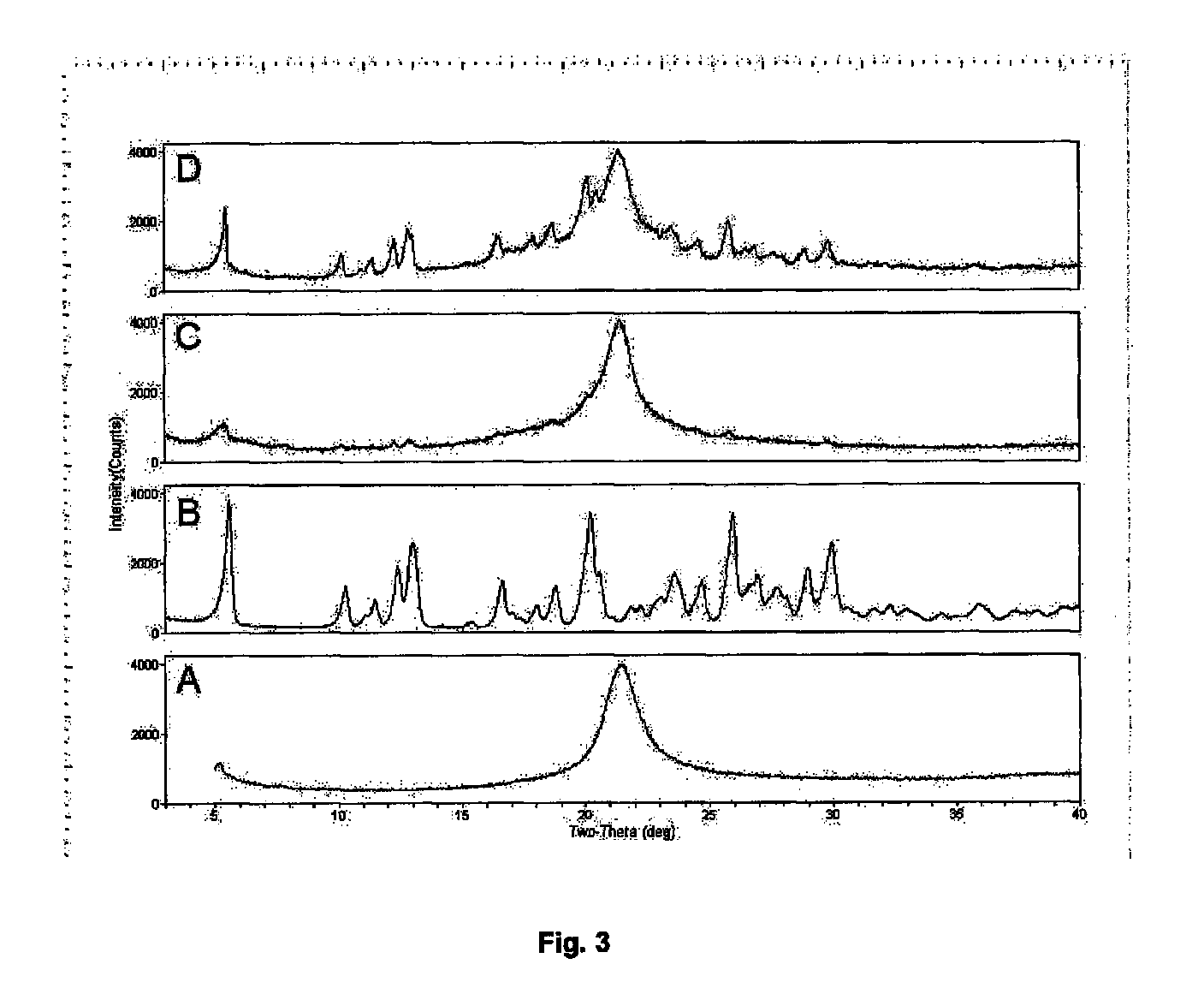
Dry powder formulations of particles that contain two or more active ingredients for treating obstructive or inflammatory airways diseases
ActiveUS20130319411A1Good chemical stabilityImprove physical stabilityBiocidePowder deliveryAdditive ingredientInhalation
Dry powder formulations for inhalation comprising spray-dried particles and their use in the treatment of an obstructive or inflammatory airways disease. Each particle has a core of a first active ingredient in substantially crystalline form that is coated with a layer of a second active ingredient in substantially amorphous form that is dispersed in a pharmaceutically acceptable hydrophobic excipient. A process for preparing such formulations is also described.
Owner:NOVARTIS AG
Novel compounds useful for the treatment of degenerative and inflammatory diseases
ActiveUS20100029709A1Prevent and treat any maladyModify activityBiocideOrganic chemistryMammalArthritis
A novel [1,2,4]triazolo[1,5-a]pyridine compound is disclosed that has a formula represented by the following:This compound may be prepared as a pharmaceutical composition, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example, diseases involving cartilage degradation, bone and / or joint degradation, for example osteoarthritis; and / or conditions involving inflammation or immune responses, such as Crohn's disease, rheumatoid arthritis, psoriasis, allergic airways disease (e.g. asthma, rhinitis), juvenile idiopathic arthritis, colitis, inflammatory bowel diseases, endotoxin-driven disease states (e.g. complications after bypass surgery or chronic endotoxin states contributing to e.g. chronic cardiac failure), diseases involving impairment of cartilage turnover (e.g. diseases involving the anabolic stimulation of chondrocytes), congenital cartilage malformations, diseases associated with hypersecretion of IL6 and transplantation rejection (e.g. organ transplant rejection) and proliferative diseases.
Owner:GALAPAGOS NV
Nebulizer Formulation
A sterile nebulizer formulation contains formoterol and budesonide in about 2 ml or less of saline and is for treatment of COPD and asthma and other airways diseases and disorders.
Owner:BREATH
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations for inhalation comprising a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of inflammatory and / or obstructive airways diseases.
Owner:CHIESI FARM SPA
Dry powder formulation comprising a corticosteroid and a beta-adrenergic for administration by inhalation
Dry powder formulations comprising a corticosteroid and a beta2-adrenergic drug in combination are useful for the prevention and / or treatment of inflammatory or obstructive airways diseases.
Owner:CHIESI FARM SPA
Vaccine compositions
InactiveUS8226959B2Organic active ingredientsBiocideAntigenStreptococcus pneumoniae capsular polysaccharide
Owner:NEWCASTE INNOVATION LTD
Vaccine compositions
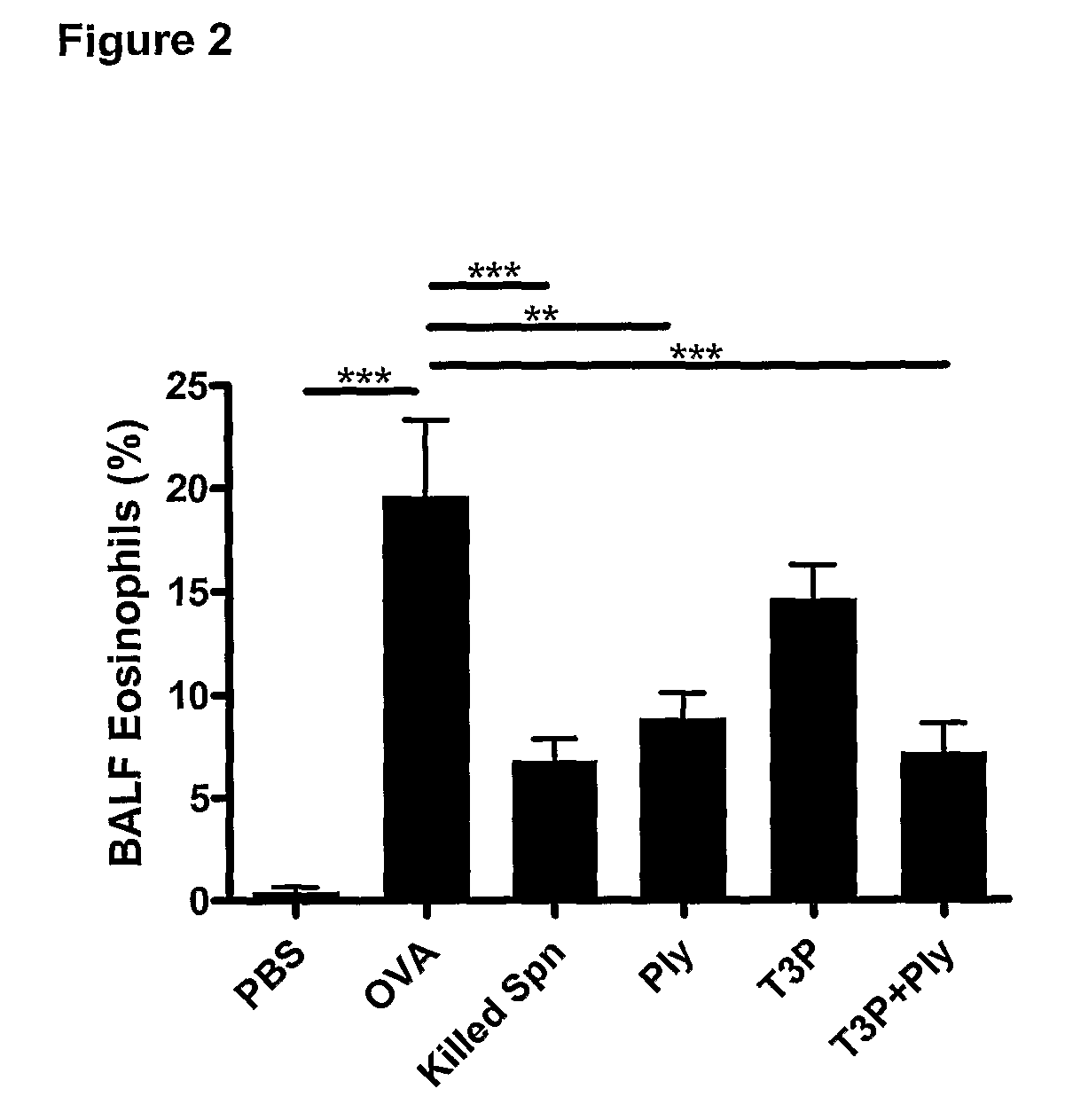
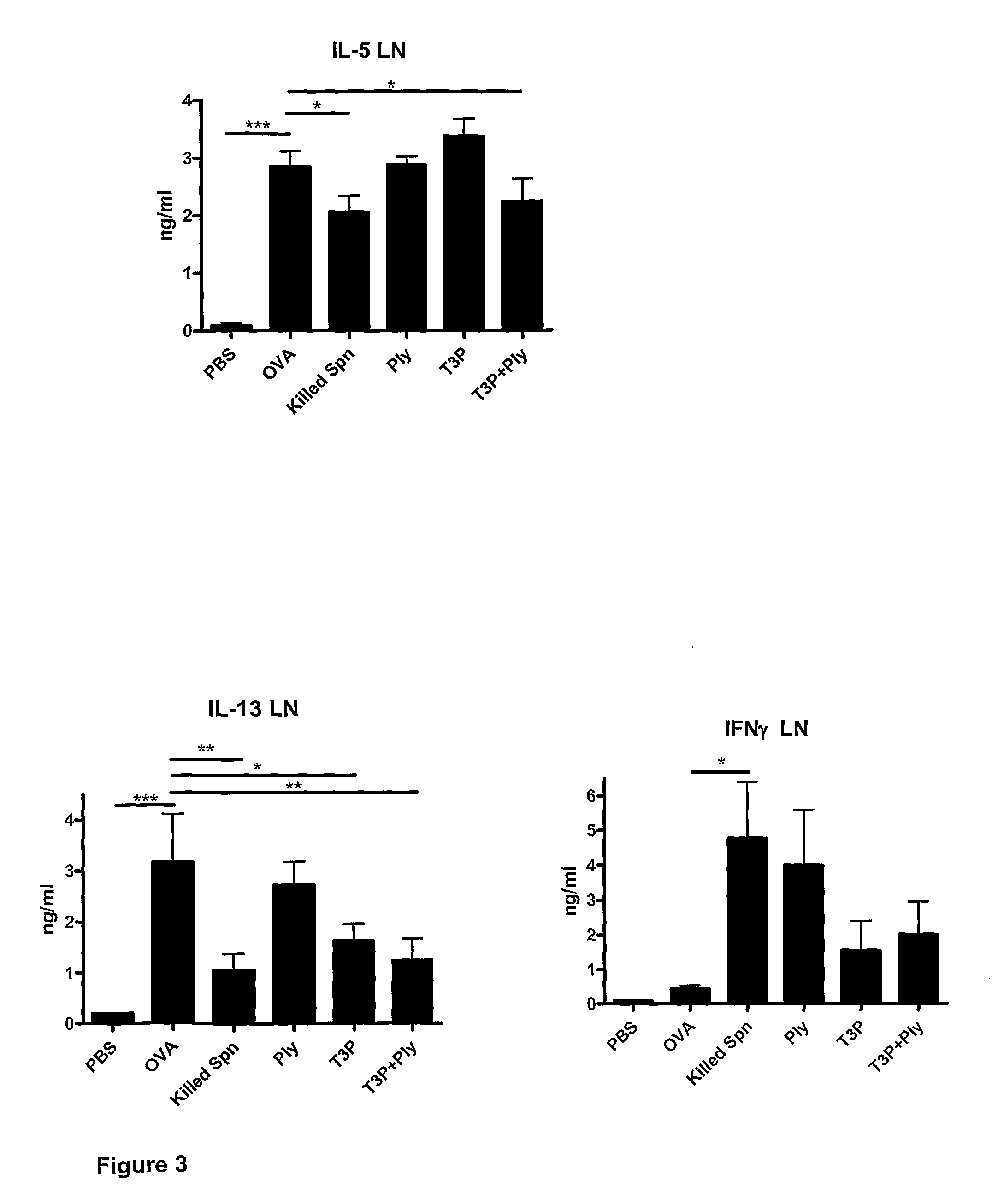
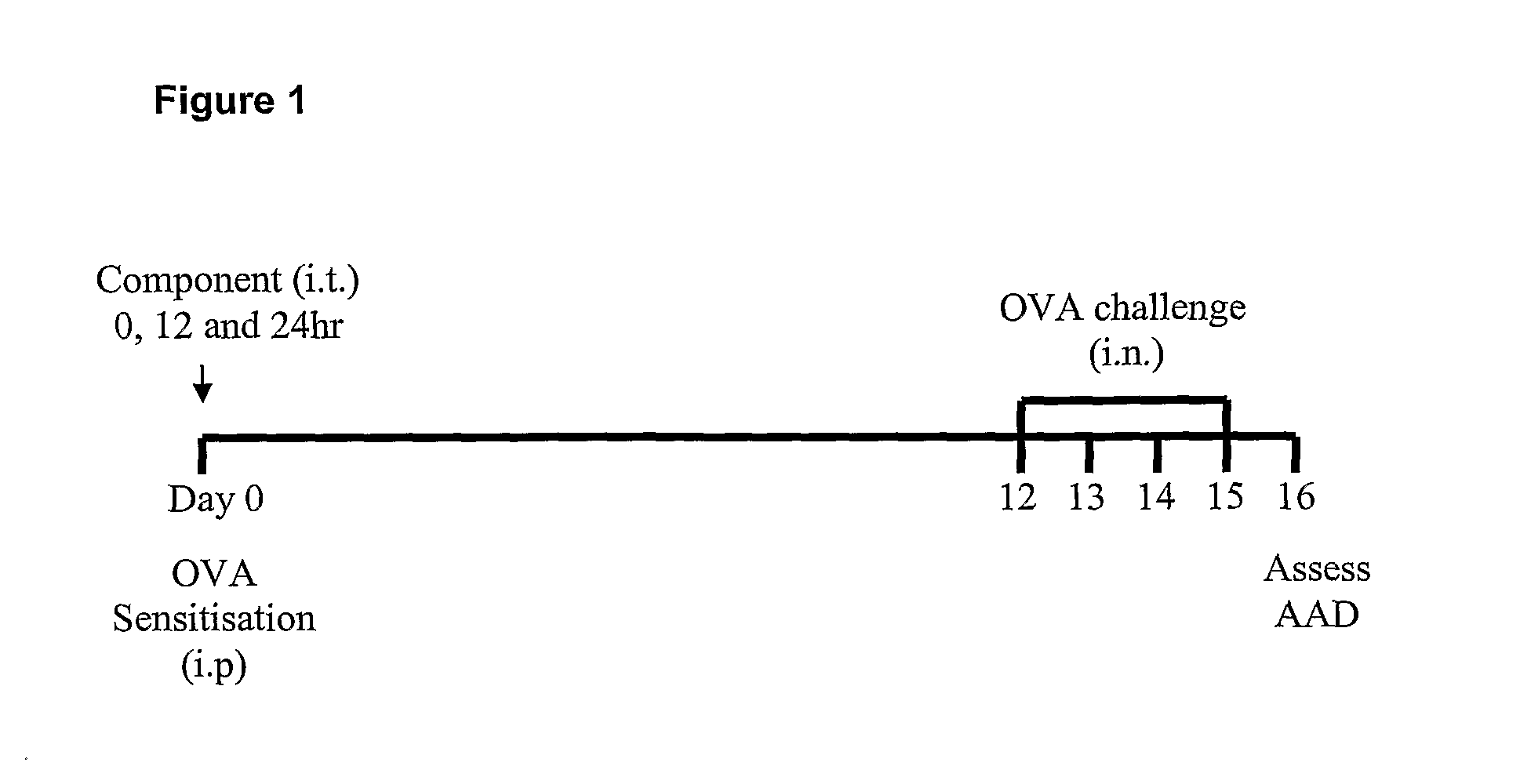
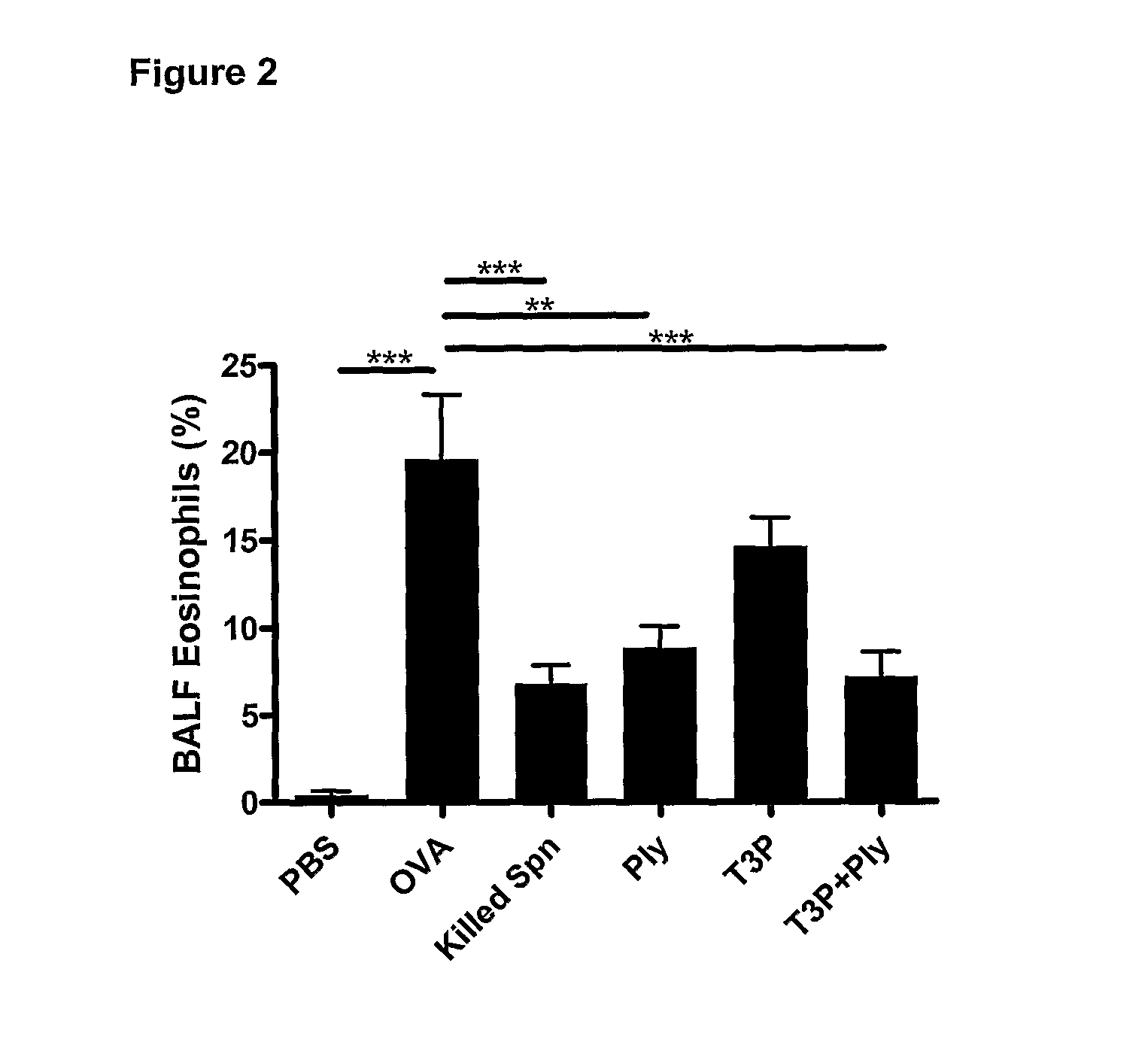
The present invention provides methods for the treatment or prevention of allergic airways diseases, the suppression of allergic immune responses, and the induction protective immunity against allergic airways diseases wherein the methods comprise administering to subjects in need thereof an effective amount of a Streptococcus pneumoniae capsular polysaccharide and a Streptococcus pneumoniae exotoxin or exotoxoid, optionally together with one or more additional antigenic or immunomodulatory constituents, components or fractions of Streptococcus pneumoniae and / or immunopotentiators. Administration of individual components is also contemplated. Also provided are vaccine compositions suitable for use in accordance with methods disclosed herein.
Owner:NEWCASTE INNOVATION LTD
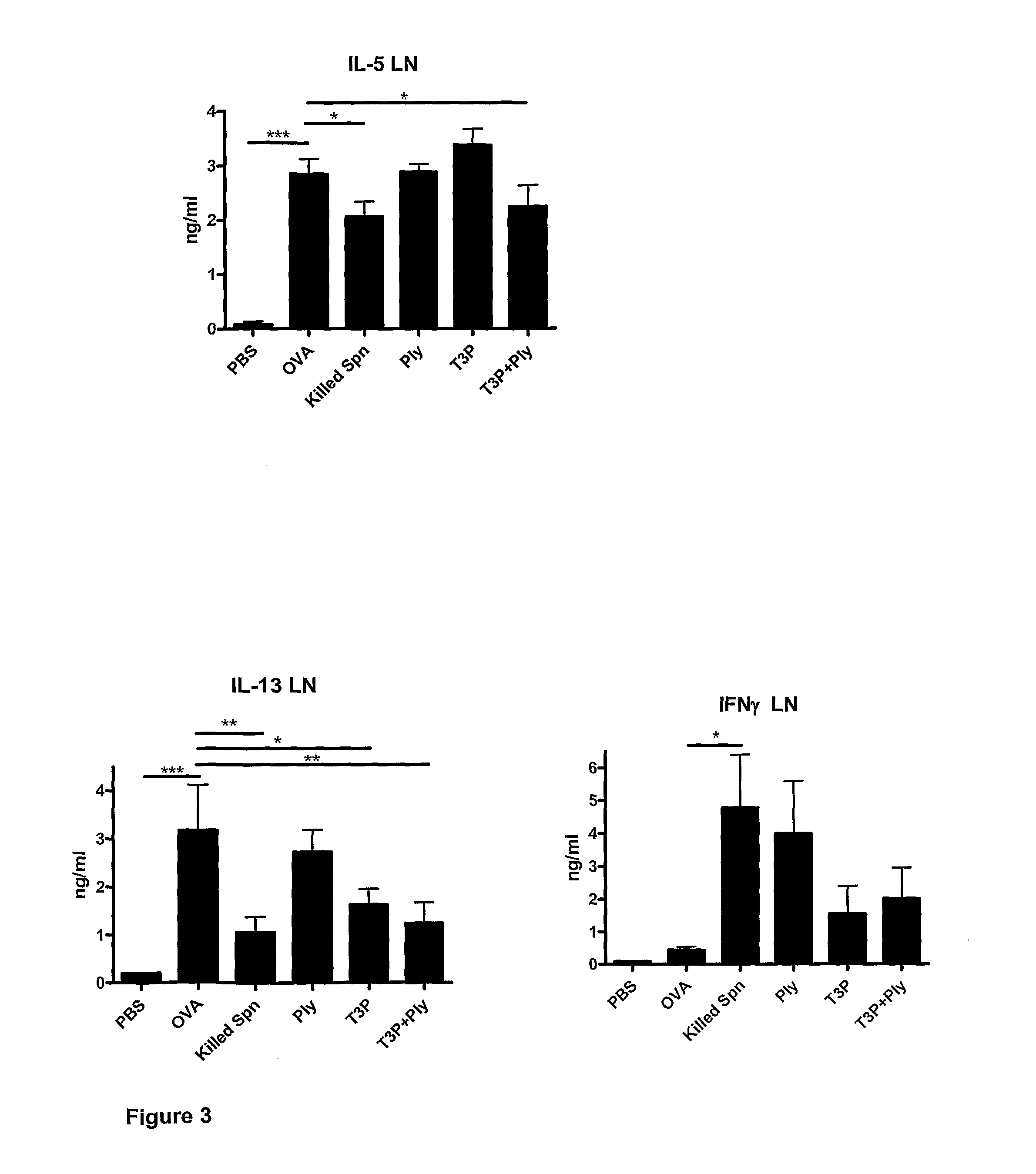
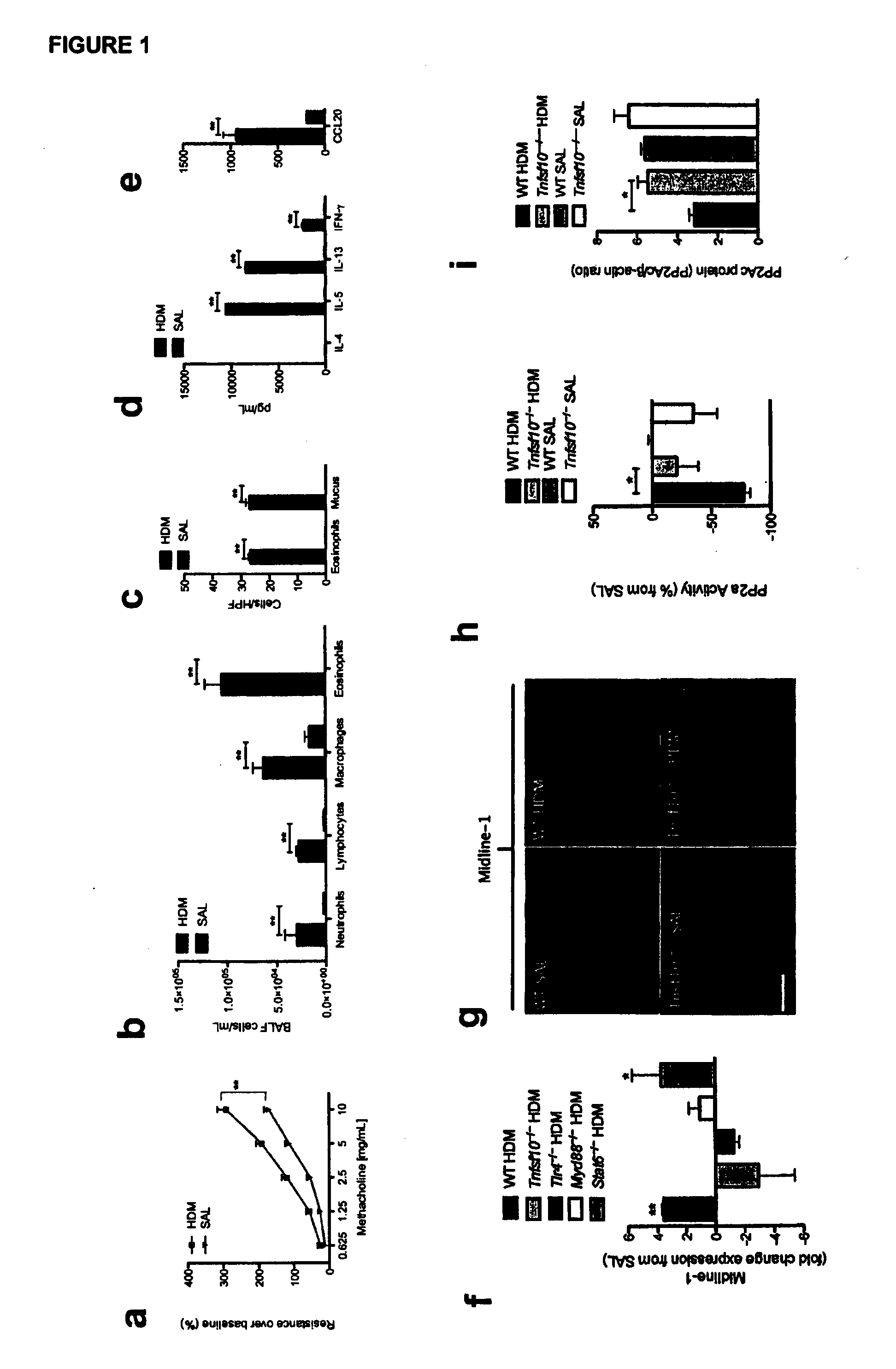
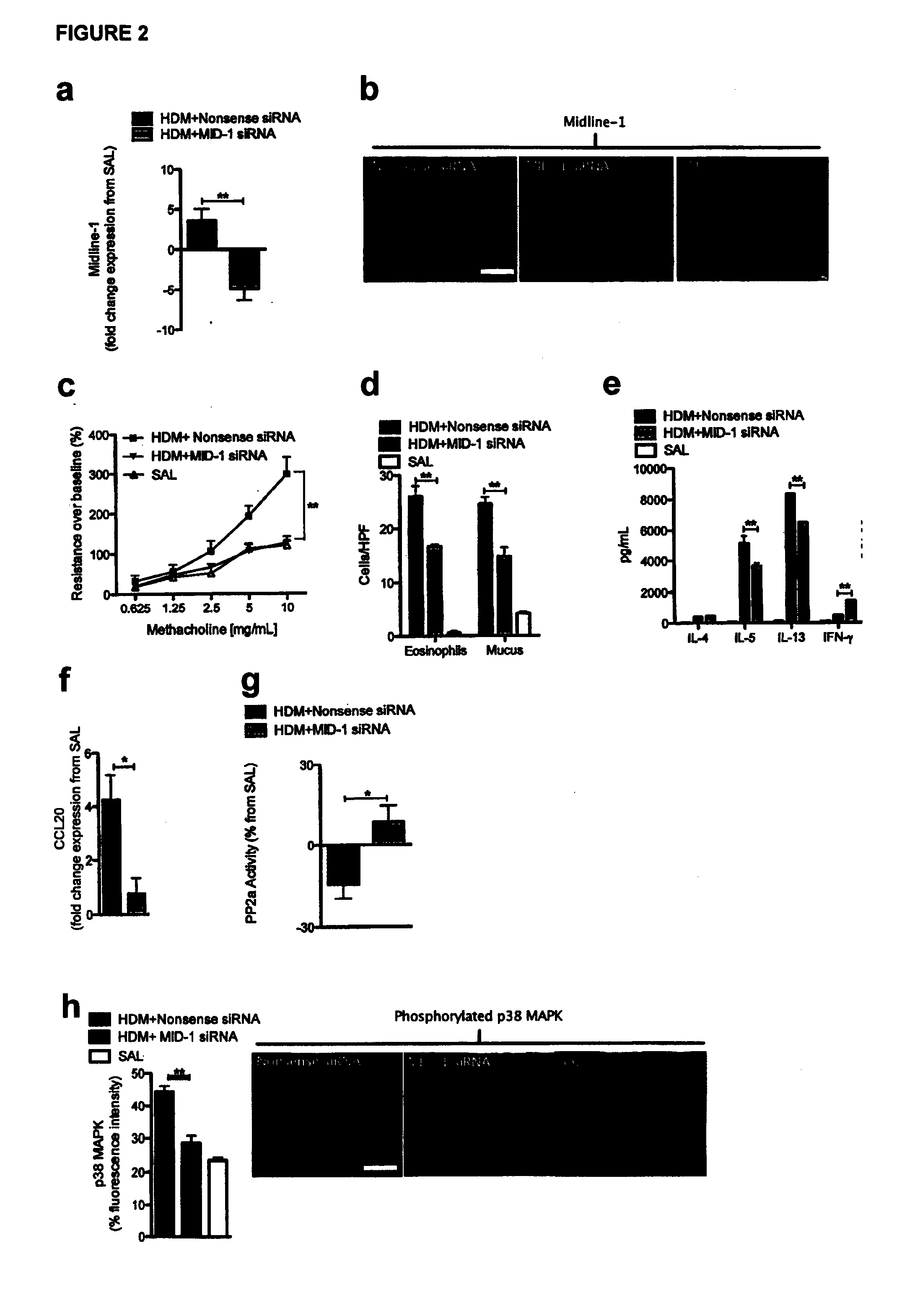
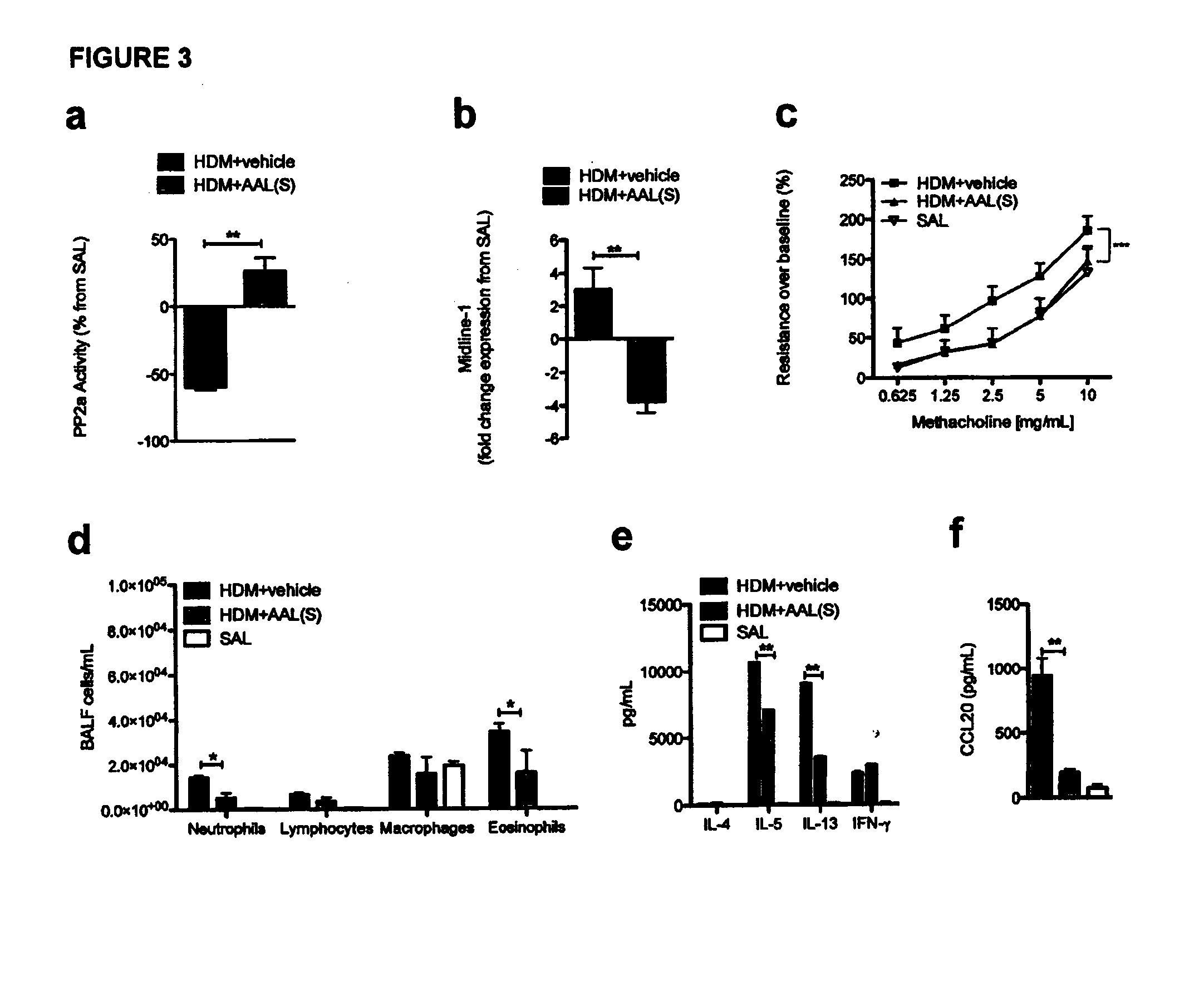
Signal transduction pathway modulation
InactiveUS20130309238A1Abolished airway hyperresponsivenessSuppressed airway inflammationCompound screeningBiocideCytokineExacerbation
Provided herein are methods and compositions for modulating signal transduction pathways by regulating the expression and / or activity of Midline-1, enabling the inhibition of airways inflammation, the inhibition of airways hyperresponsiveness, the inhibition of rhinovirus-associated inflammation, and reductions in cytokine and chemokine release. Methods and compositions disclosed herein facilitate the treatment and prevention of conditions associated with airway inflammation, airway tissue remodelling and rhinovirus-associated inflammation and symptoms, manifestations and exacerbations thereof, in particular of allergic diseases such as allergic airways diseases including asthma.
Owner:NEWCASTE INNOVATION LTD
Dry powder inhalers comprising a carrier other than lactose
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Dry powder inhalers comprising a carrier other than lactose and a ternary component
This invention relates to novel pharmaceutical compositions for inhalation comprising separately, sequentially or together, drugs having amine in the form of a dry powder in admixture with a pharmaceutically acceptable carrier, other than lactose, and its use in the treatment of respiratory condition selected from asthma and chronic obstructive pulmonary disease (COPD) and other obstructive airways diseases. More particularly, the invention relates to pharmaceutical composition for inhalation further comprising a ternary component. In addition, the present invention relates to novel pharmaceutical composition for inhalation based on combinations of long acting muscarinic antagonists, long acting beta agonists, short acting beta-2 agonists, corticosteroids or a combination of two or more of them.
Owner:ARVEN ILAC SANAYI VE TICARET
Novel compounds useful for the treatment of degenerative and inflammatory diseases
ActiveUS20110190260A1Prevent and treat any maladyModify activityBiocideOrganic chemistryArthritisOsteocyte
[1,2,4]Triazolo[1,5-a]pyridine compounds are disclosed that have a formula represented by the formula (I). The compounds may be prepared as pharmaceutical compositions, and may be used for the prevention and treatment of a variety of conditions in mammals including humans, including by way of non-limiting example, diseases involving cartilage degradation, bone and / or joint degradation, for example osteoarthritis; and / or conditions involving inflammation or immune responses, such as Crohn's disease, rheumatoid arthritis, psoriasis, allergic airways disease (e.g. asthma, rhinitis), juvenile idiopathic arthritis, colitis, inflammatory bowel diseases, endotoxin-driven disease states (e.g. complications after bypass surgery or chronic endotoxin states contributing to e.g. chronic cardiac failure), diseases involving impairment of cartilage turnover (e.g diseases involving the anabolic stimulation of chondrocytes), congenital cartilage malformations, diseases associated with hypersecretion of IL6, transplantation rejection (e.g. organ transplant rejection) and proliferative diseases.
Owner:GALAPAGOS NV
Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid
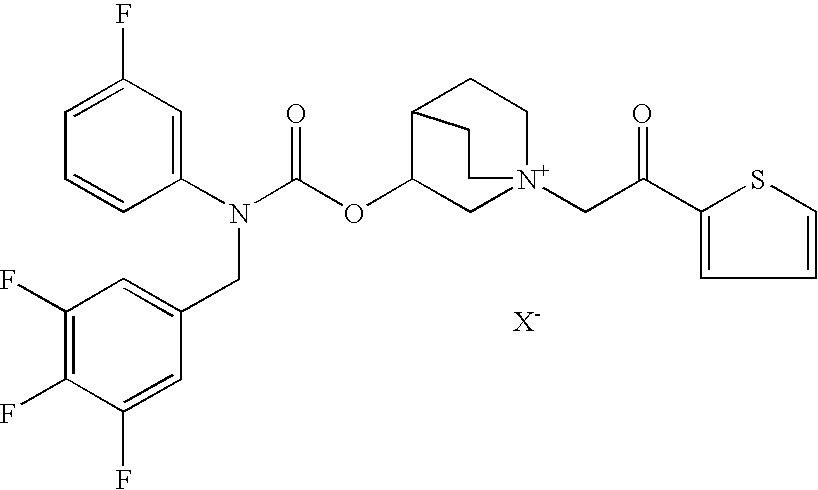
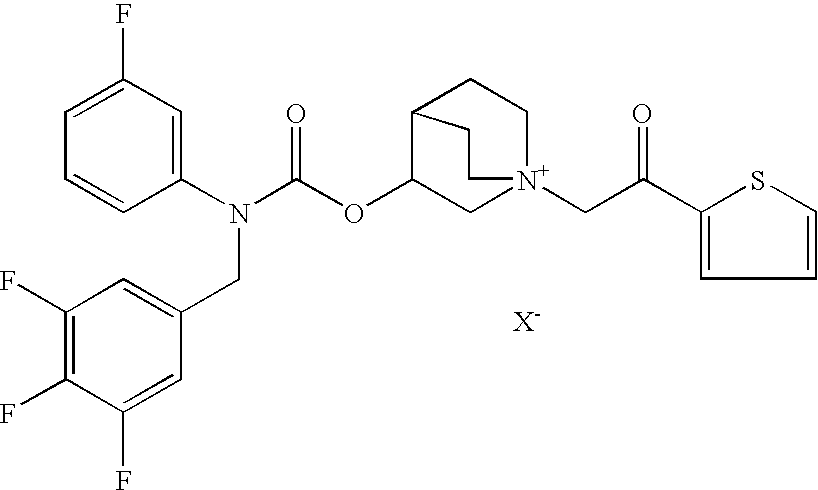
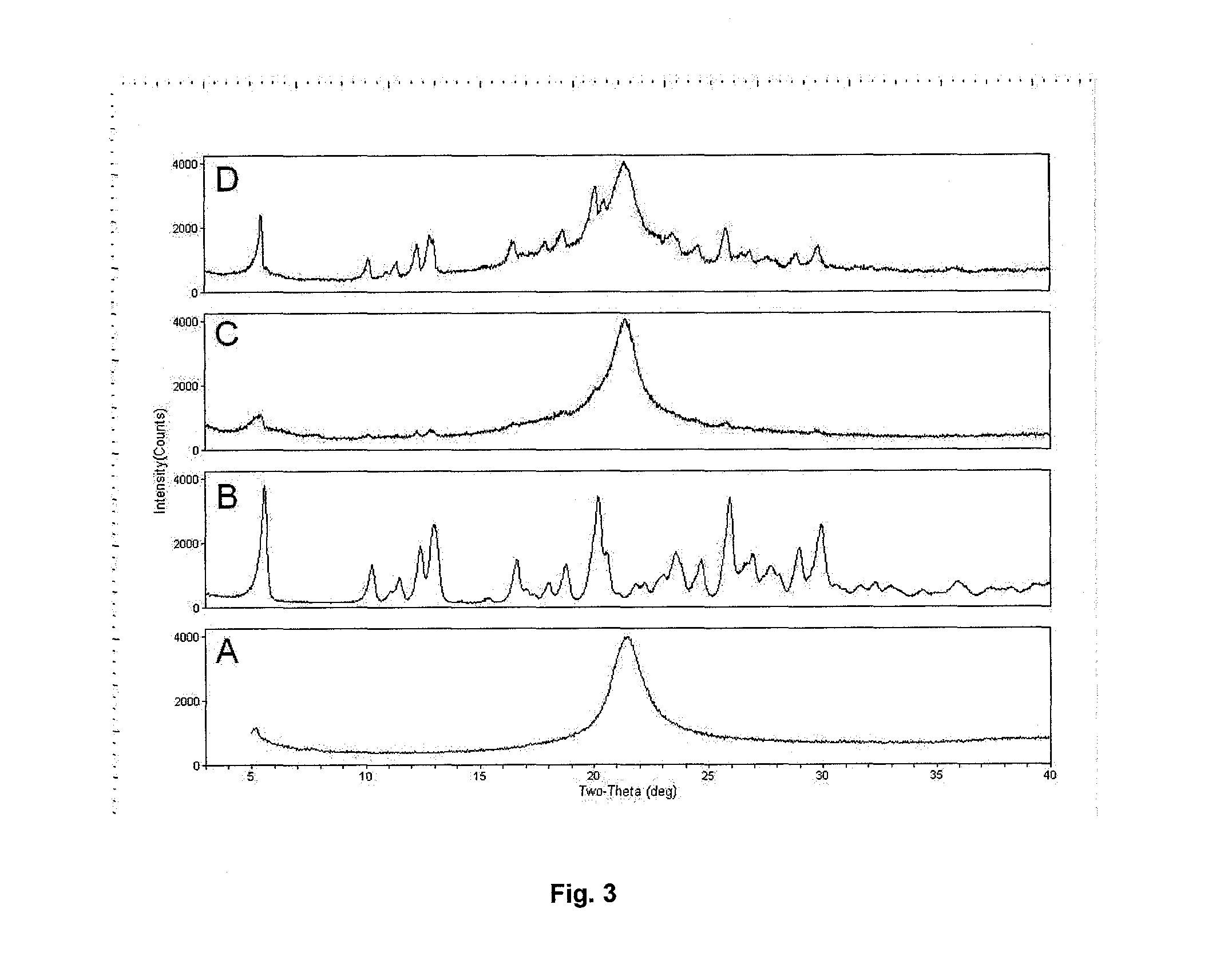
InactiveUS20100269825A1Improve propertiesGood benefitBiocideOrganic active ingredientsInhalationMethyl group
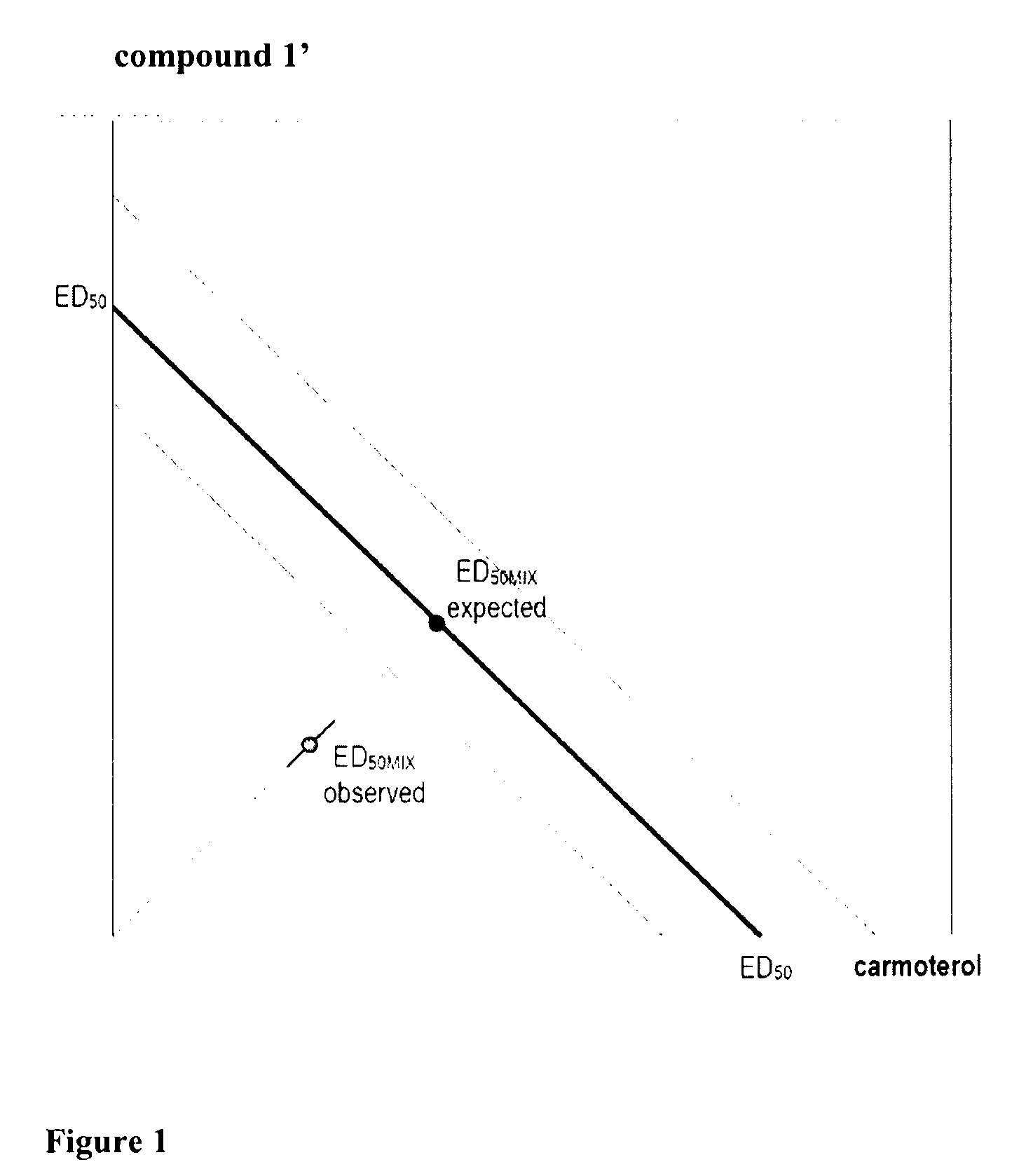
Crystalline particles wherein each particle comprises a combination of a pharmaceutically acceptable salt of 8-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino]ethyl]-2(1H)-quinolinone (carmoterol), and a corticosteroid in a pre-determined and constant ratio are effective for the prevention and treatment of inflammatory or obstructive airways diseases.
Owner:CHIESI FARM SPA
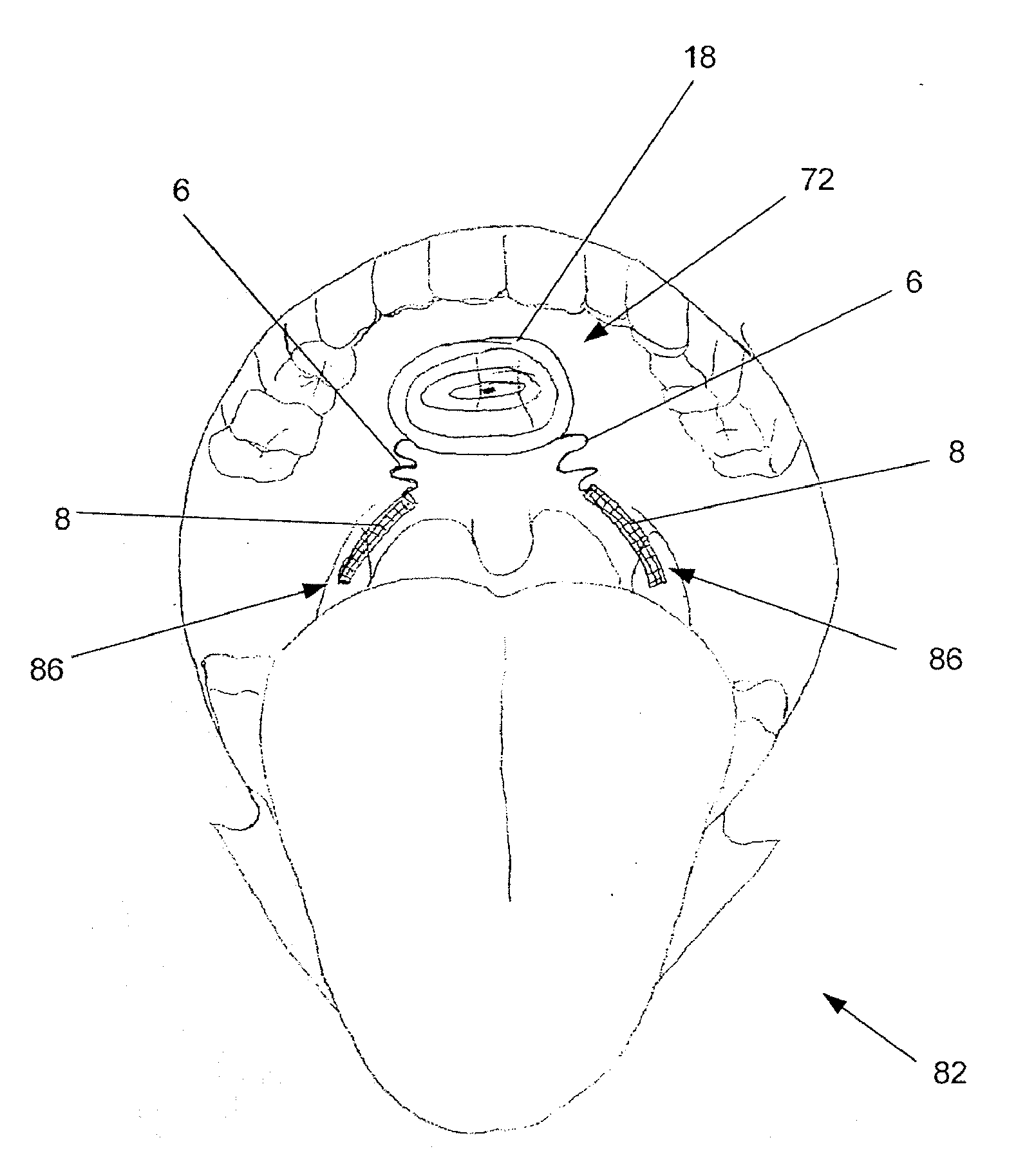
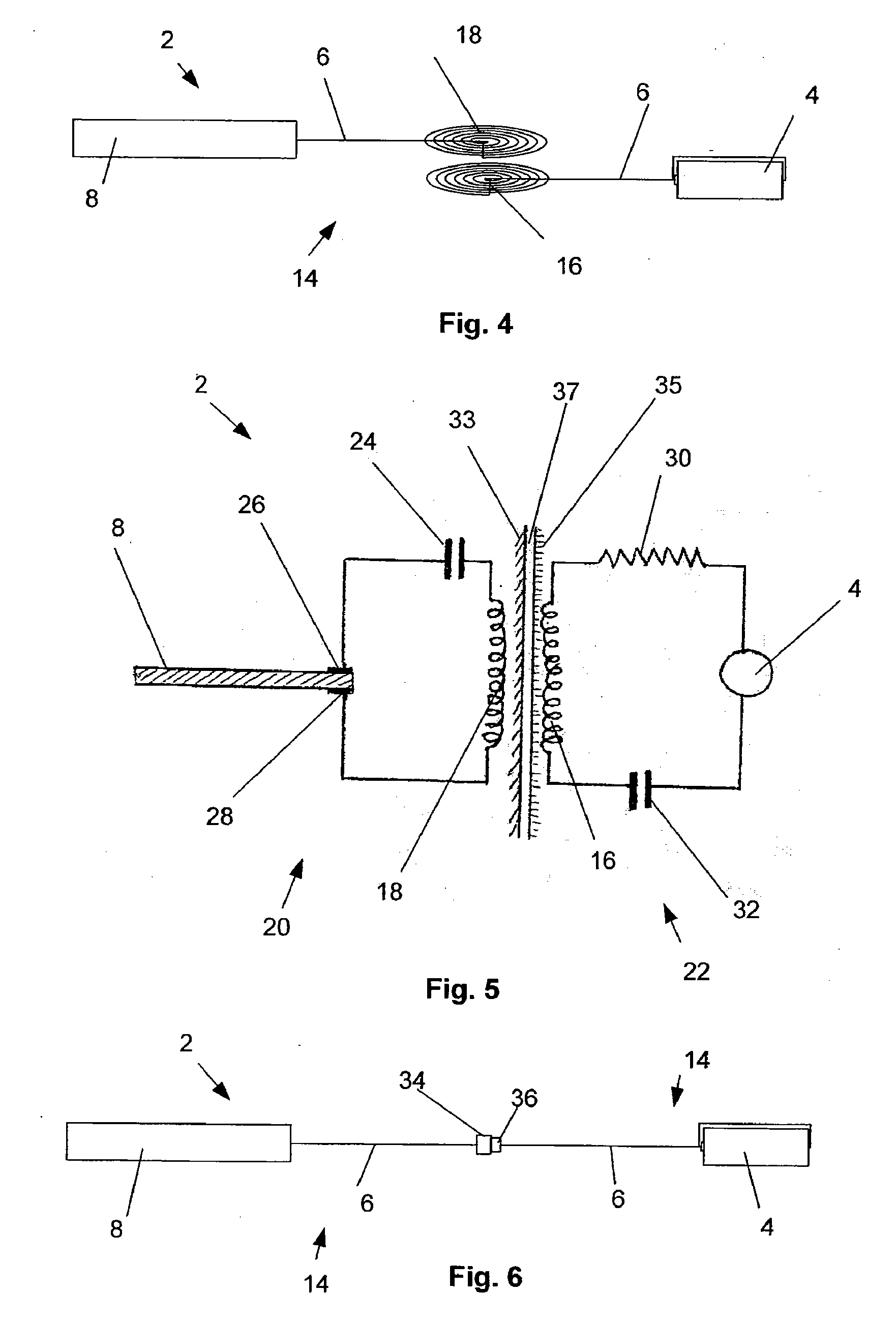
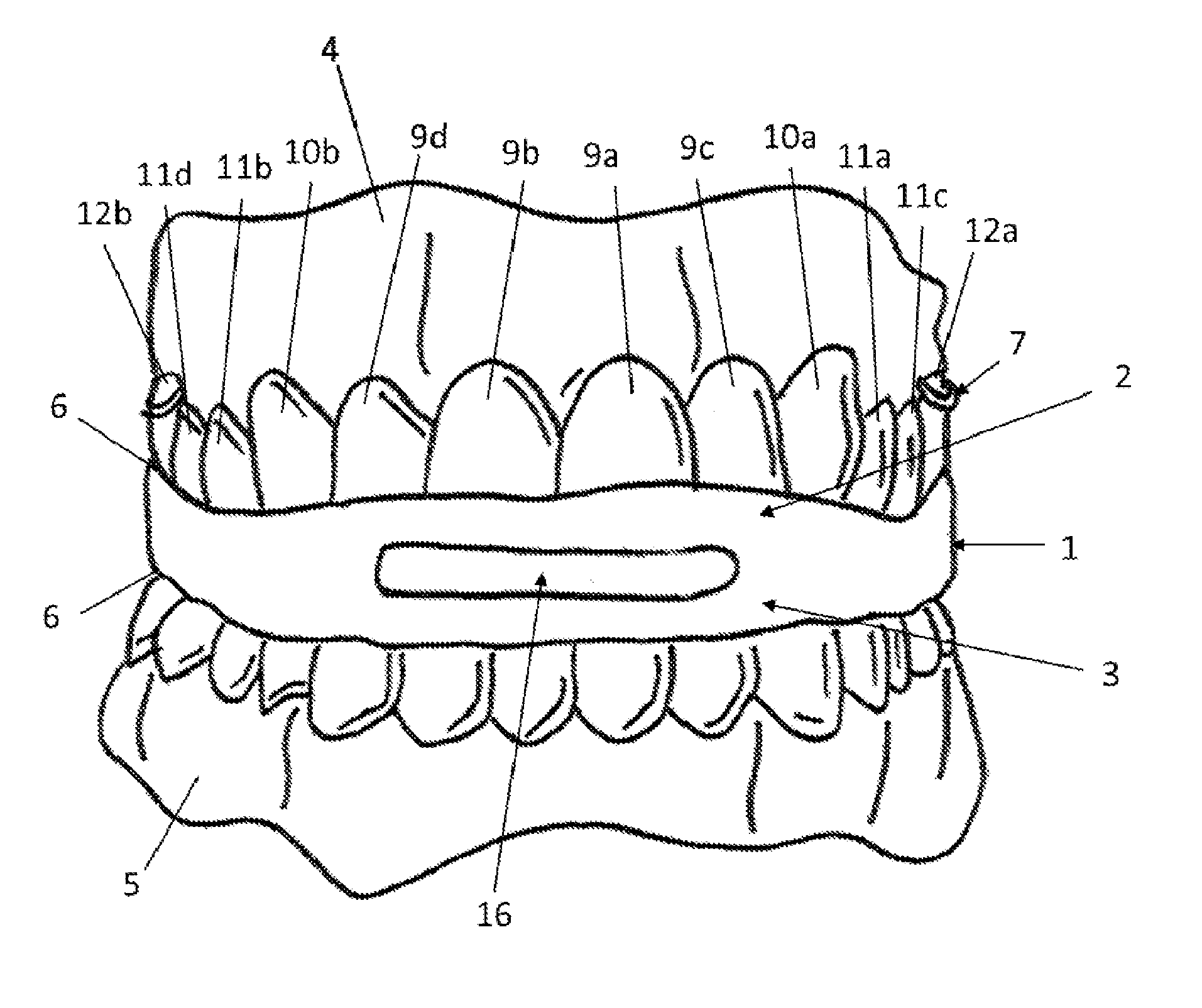
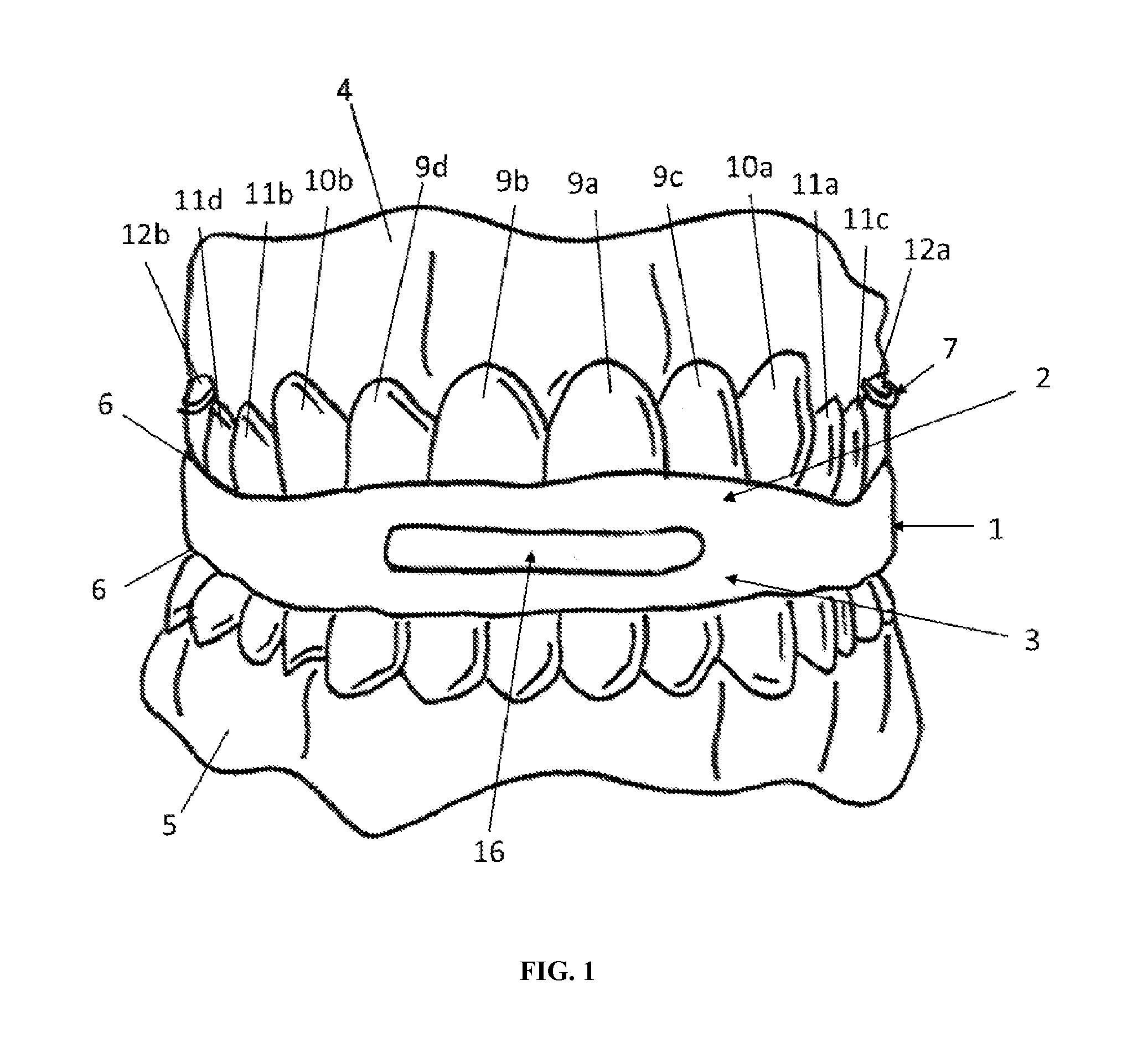
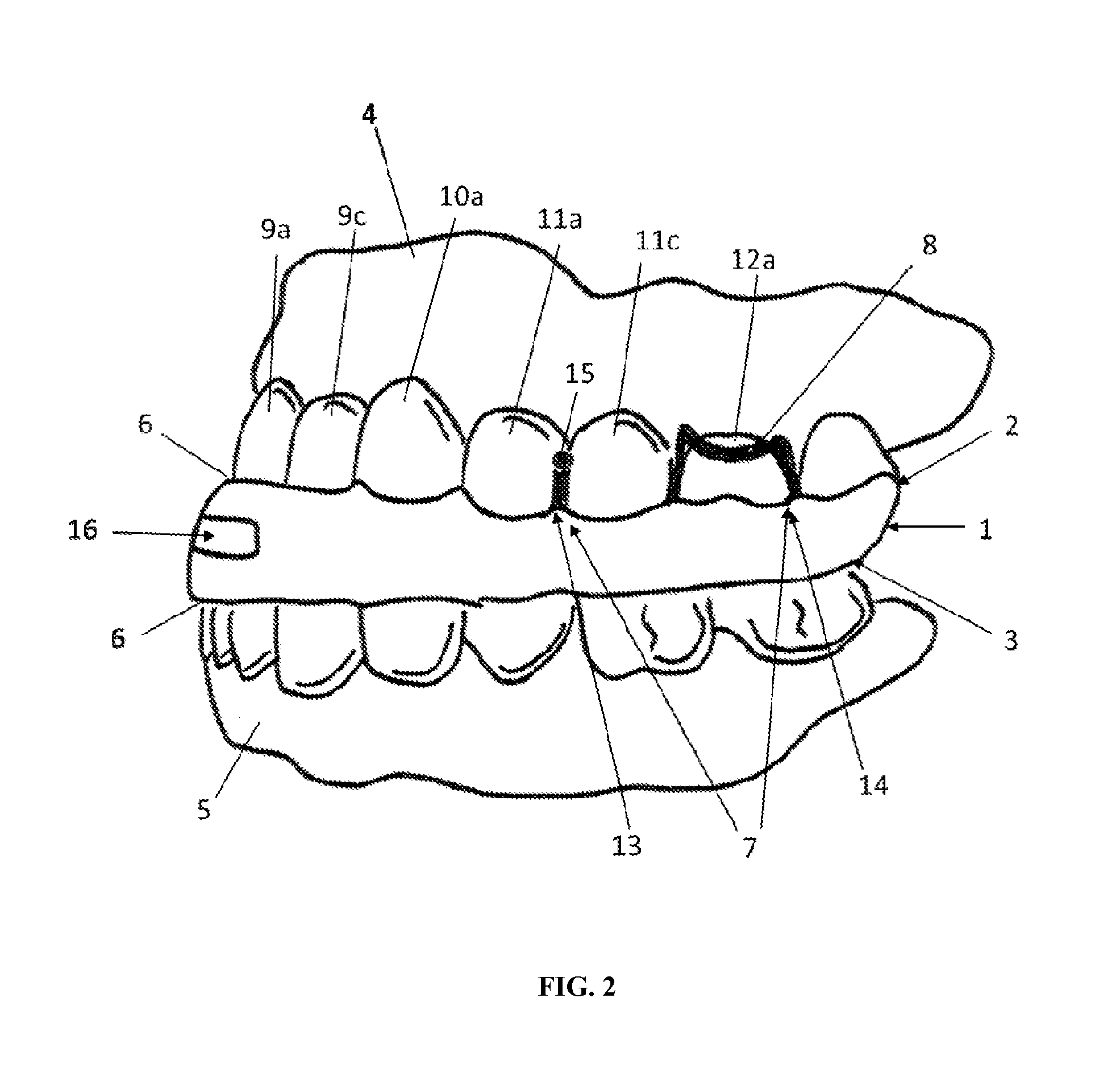
Intraoral functional device for relieving obstructive sleep apnea syndrom, snoring and/or other airway disorders
An intraoral device (1) includes upper cooperating elements (2) adapted to cooperate with the upper jaw (4) and lower cooperating elements (3) adapted to cooperate with the lower jaw (5); a bracket (19) fixed in the cooperating elements (2,3), the bracket (19) supports a target (18) aiming at inducing mandibular advancement and attracting the tip of the tongue; the target (18) being centered, in a transverse plane, with regard to the cooperating elements (2,3). The device (1) further includes stimulating elements (17) for stimulation of the tongue muscles, preferably the genioglossus, involving reflex exploration of the stimulating elements (17) by the tongue. The device (1) may be used for relieving obstructive sleep apnea syndrome, snoring and / or other airway disorders.
Owner:PANTHERA DENTAIRE INC
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
Dry powder formulations for inhalation containing a combination of an anti-cholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of an inflammatory and / or obstructive airways disease.
Owner:CHIESI FARM SPA
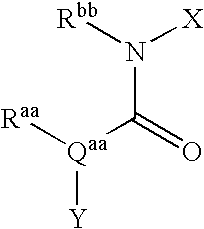
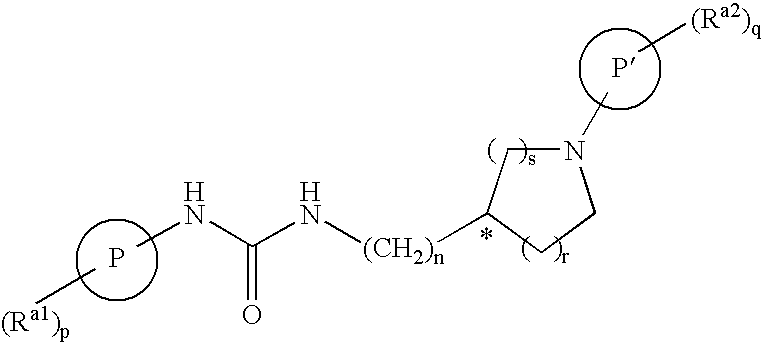
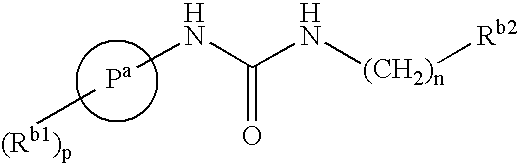
Bicyclic Amide, Carbamate or Urea Derivatives as Vanilloid Receptor Modulators
This invention relates to bicyclic amide, carbamate or urea derivatives and salts thereof which are useful as active ingredients of pharmaceutical preparations. The bicyclic amide, carbamate or urea derivative of the present invention has vanilloid receptor (VR1) antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with VR1 activity, in particular for the treatment of urological diseases or disorders, such as detrusor overactivity (overactive bladder), urinary incontinence, neurogenic detrusor oeractivity (detrusor hyperflexia), idiopathic detrusor overactivity (detrusor instability), benign prostatic hyperplasia, and lower urinary tract symptoms; chronic pain, neuropathic pain, postoperative pain, rheumatoid arthritic pain, neuralgia, neuropathies, algesia, nerve injury, ischaemia, neurodegeneration, stroke, and inflammatory disorders such as asthma and chronic obstructive pulmonary (or airways) disease (COPD).
Owner:BAYER SCHERING PHARMA AG
Dry powder formulations of particles that contain two or more active ingredients for treating obstructive or inflammatory airways diseases
ActiveUS9050267B2Improved lung targetingImprove delivery efficiencyPowder deliverySpray deliveryDiseaseInhalation
Dry powder formulations for inhalation comprising spray-dried particles and their use in the treatment of an obstructive or inflammatory airways disease. Each particle has a core of a first active ingredient in substantially crystalline form that is coated with a layer of a second active ingredient in substantially amorphous form that is dispersed in a pharmaceutically acceptable hydrophobic excipient. A process for preparing such formulations is also described.
Owner:NOVARTIS AG
Protein biomarkers for obstructive airways diseases
InactiveUS20130183684A1Accurate diagnosisDisease diagnosisBiological testingObstructive airway diseaseImmunoglobulin A
Provided herein are methods for the diagnosis of obstructive airways diseases such as asthma and chronic obstructive pulmonary disease, and the discrimination between such diseases based on the expression profiles of biomarker proteins and combinations of biomarker proteins. In particular embodiments the biomarker proteins are selected from ceruloplasmin, haptoglobin, hemopexin, -2-macroglobulin, prothrombin, immunoglobulin A and complement factor H.
Owner:NEWCASTE INNOVATION LTD
Acetamide Stereoisomer
The compound of formula (I)is a water-stable, long acting β2-selective adrenoceptor agonist useful as a bronchodilator in the treatment of bronchoconstriction associated with reversible obstructive airways diseases and the like.
Owner:SUNOVION PHARMA INC
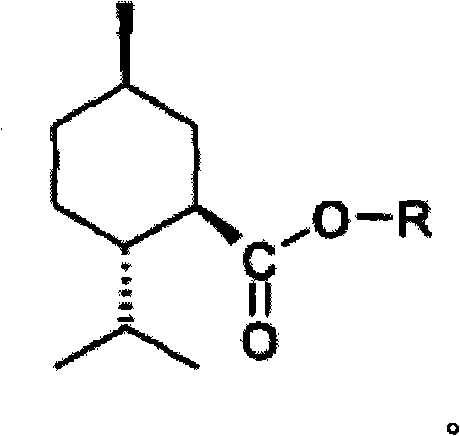
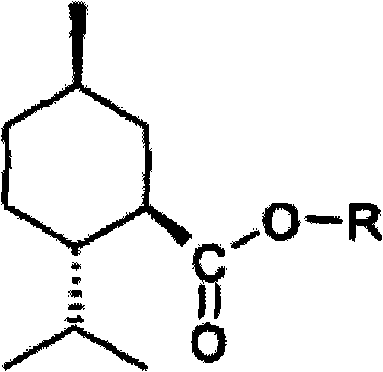
P-menthawe-3-carboxylic acid esters to treat airways diseases
The present invention relates to methods and devices for treating sensory discomfort in the upper airways of a human; treating sensory discomfort in the oropharynx of a human; alleviating pain from pharyngitis in a human; alleviating cough in a human; and ameliorating the symptoms and signs of asthma, dyspnea, sleep apnea, snoring, or chronic obstructive pulmonary disease in a human. According to the invention, an active compound is delivered, preferably selectively delivered, to the oropharynx, preferably the oropharyngeal surfaces, more preferably the lower retropalatal oropharynx (LRO) of the patient. According to the invention, the active compound is a compound of the following formula, wherein -R is C2 to C4 hydroxyalkyl or polyhydroxyalkyl.
Owner:爱德华·塔克·韦
Nebulizer Formulation
A nebulizer formulation contains levalbuterol and ipratropium in about 2 ml or less of saline and is used for treatment of COPD and asthma and other airways diseases and disorders with increased patient compliance.
Owner:BREATH
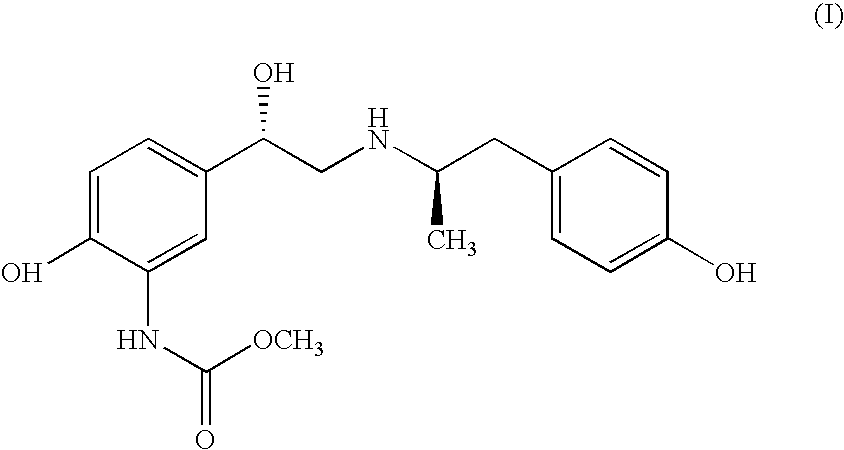
Carbamate Stereoisomer
ActiveUS7718822B2Maintain good propertiesLow affinityBiocideCarbamic acid derivatives preparationMedicineAirways disease
The compound of formula (I)is a water-stable, long acting β2-selective adrenoceptor agonist useful as a bronchodilator in the treatment of bronchoconstriction associated with reversible obstructive airways diseases and the like.
Owner:SUNOVION PHARMA INC
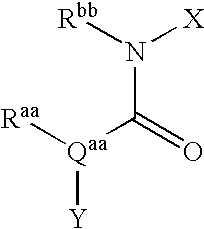
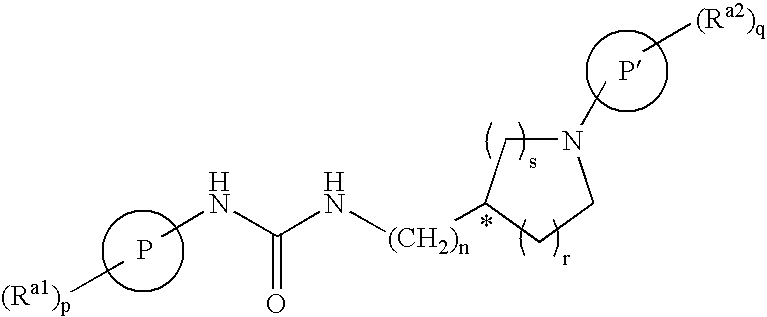
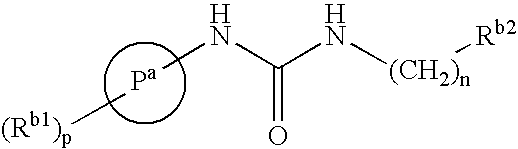
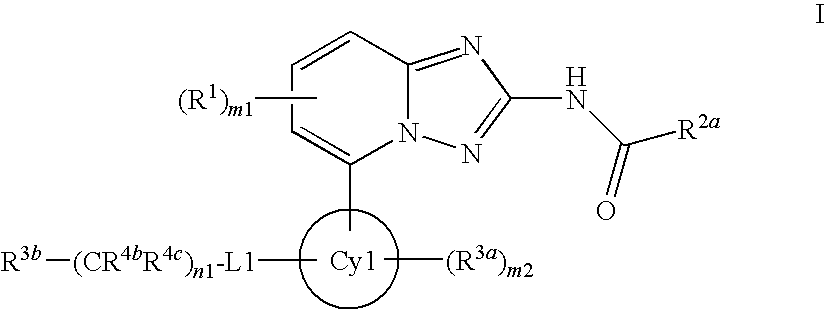
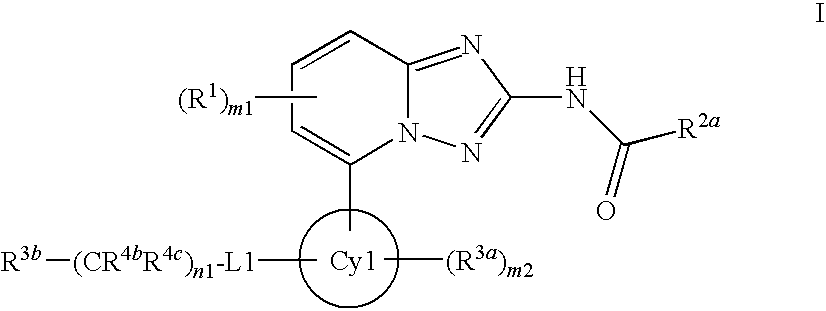
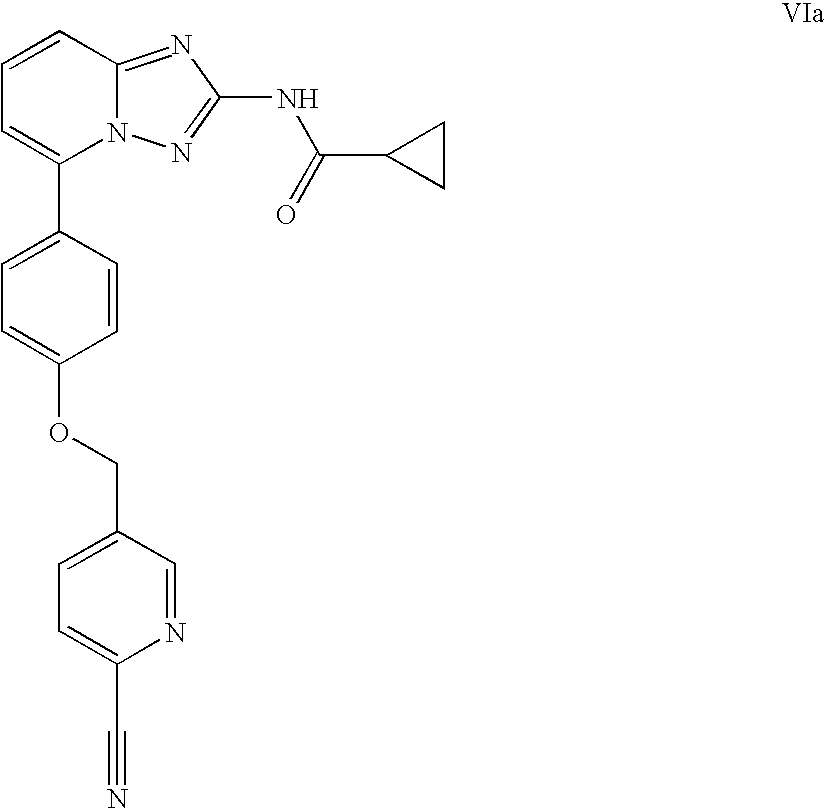
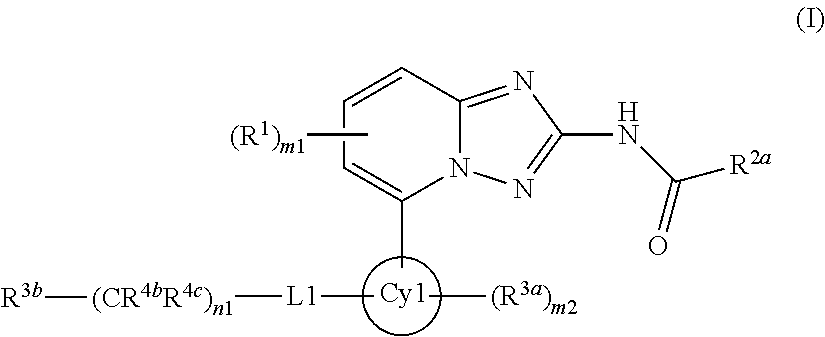
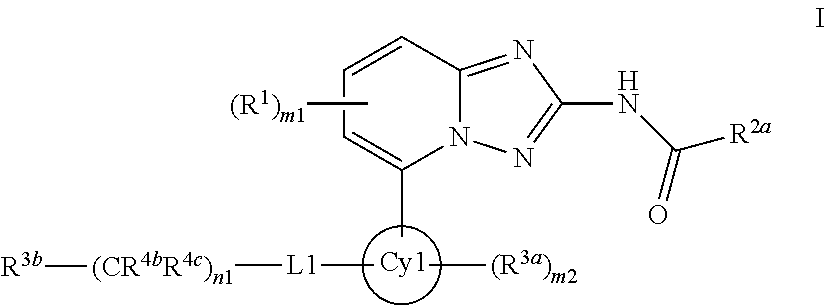
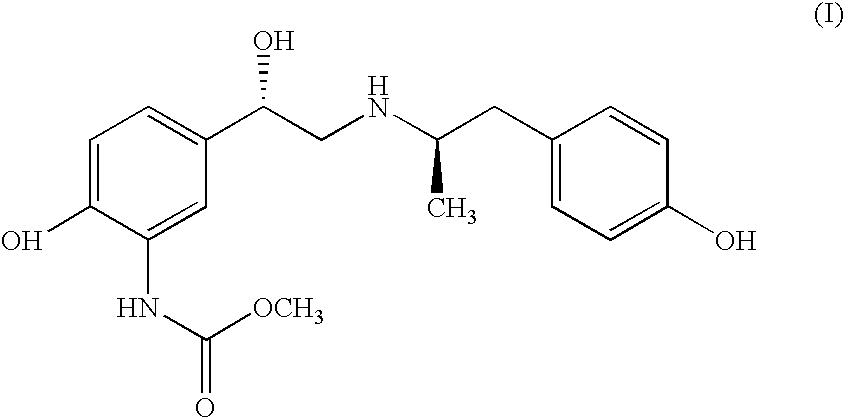
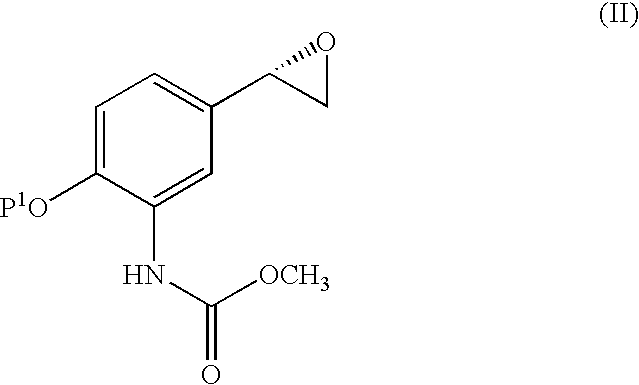
Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors
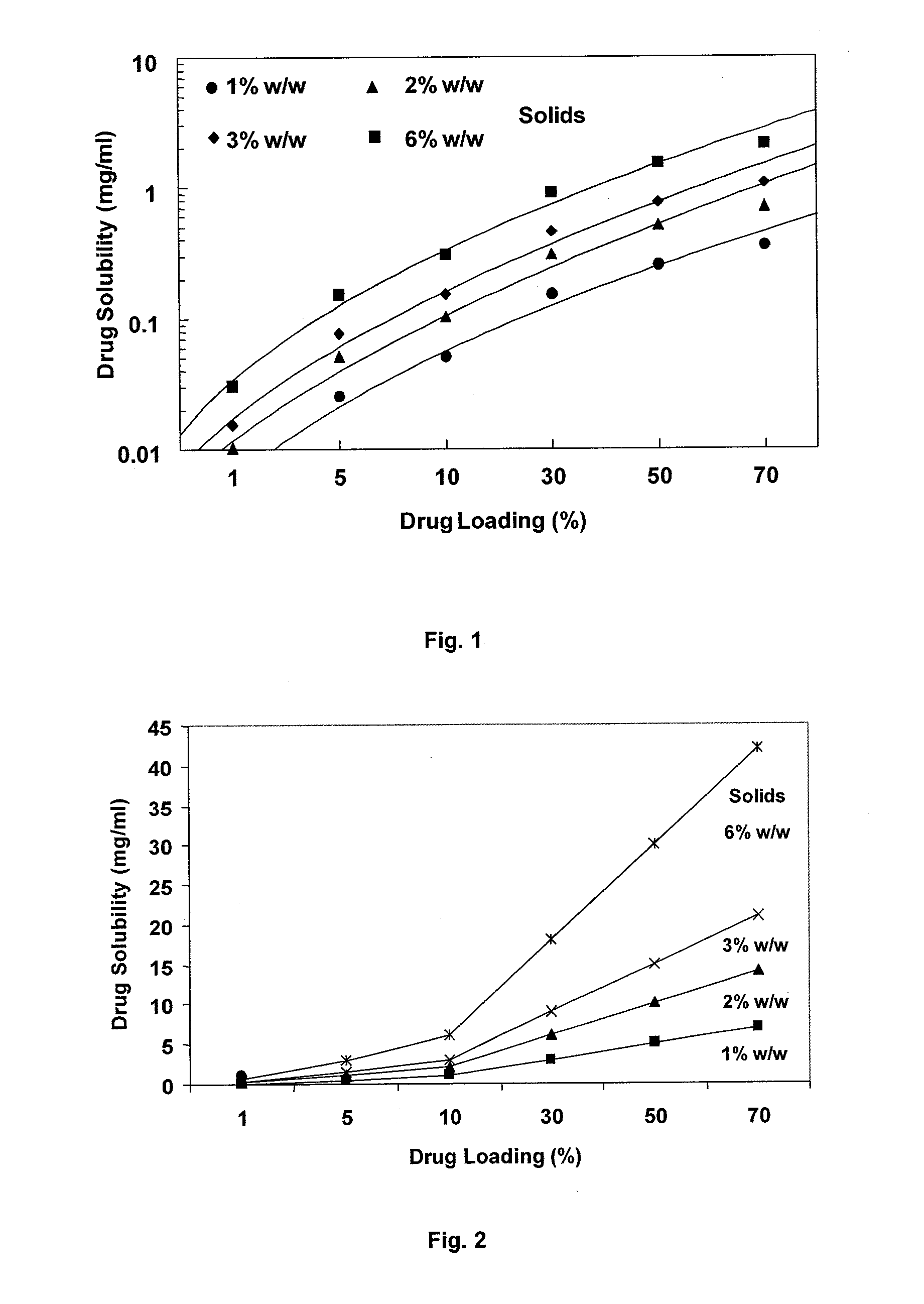
ActiveUS9573949B2Organic active ingredientsOrganic chemistryDiseaseRESPIRATORY DISTRESS SYNDROME ADULT
Mitogen activated protein kinase (MAPK) inhibitors disclosed herein are useful for the treatment of diseases of the respiratory tract, such as chronic eosinophilic pneumonia, asthma, COPD, adult respiratory distress syndrome (ARDS), exacerbation of airways hyper-reactivity consequent to other drug therapy, and airways disease that is associated with pulmonary hypertension.
Owner:CHIESI FARM SPA
Nebulizer formulation and production method thereof
A sterile nebulizer formulation contains formoterol and budesonide in about 2ml or less of saline and is for treatment of COPD and asthma and other airways diseases and disorders.
Owner:BREATH
Process for preparing a dry powder formulation comprising an anticholinergic, a corticosteroid and a beta-adrenergic
Dry powder formulations for inhalation containing a combination of an anticholinergic, a long-acting beta2-adrenoceptor agonist, and a corticosteroid are useful for the prevention and / or treatment of an inflammatory and / or obstructive airways disease.
Owner:CHIESI FARM SPA
Pharmaceutical formulation comprising an anticholinergic drug
InactiveUS20090180969A1Significant comprehensive benefitsOrganic active ingredientsDispersion deliveryAnticholinergic DrugsPharmaceutical formulation
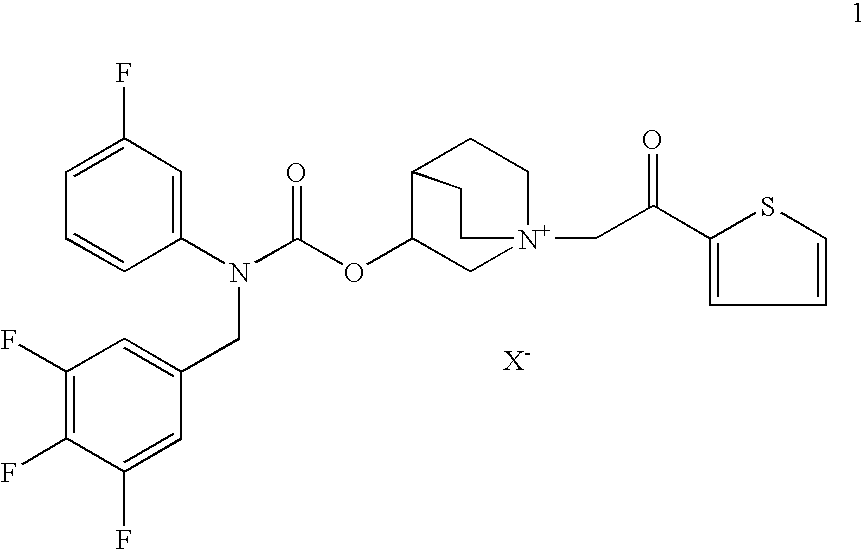
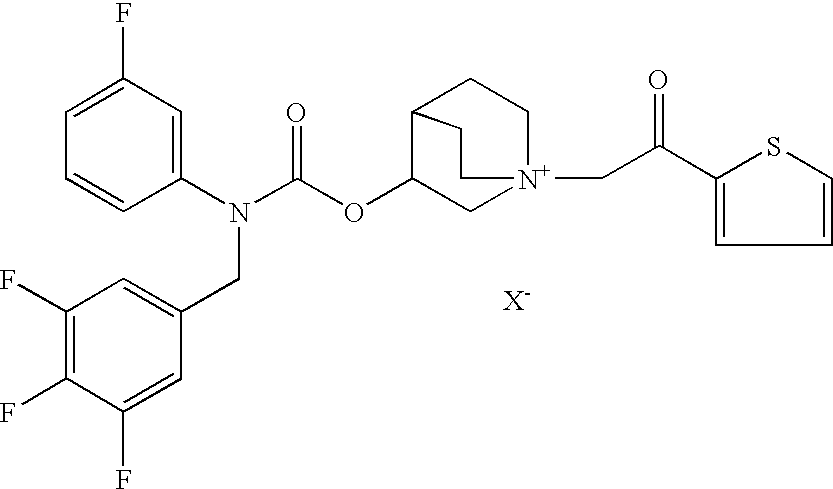
Pharmaceutical formulations suitable to be administered by pressurised metered dose inhalers (pMDIs) comprising a salt of 3-[[[(3-fluorophenyl)[(3,4,5-trifluoro phenyl)methyl]amino]carbonyl]oxy]-1-[2-oxo-2-(2-thienyl)ethyl]-1-azoniabicyclo[2.2.2]octane are effective for the prevention and / or treatment of an obstructive airways disease.
Owner:CHIESI FARM SPA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

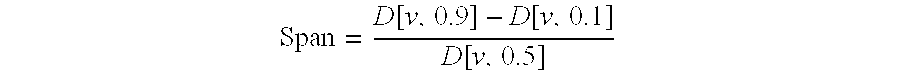
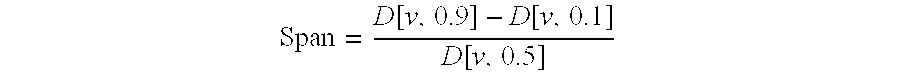
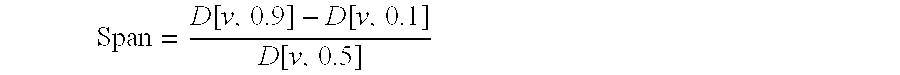
![Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid](https://images-eureka.patsnap.com/patent_img/32424778-f83f-4807-814b-f6bddf6c9310/US20100269825A1-20101028-D00000.png)
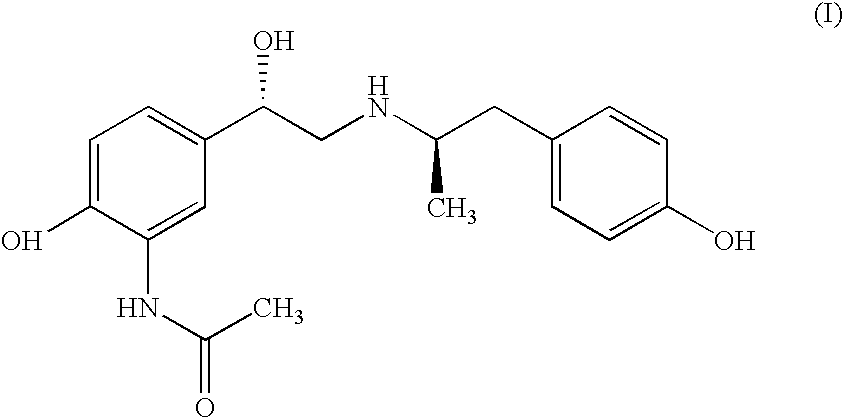
![Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid](https://images-eureka.patsnap.com/patent_img/32424778-f83f-4807-814b-f6bddf6c9310/US20100269825A1-20101028-D00001.png)
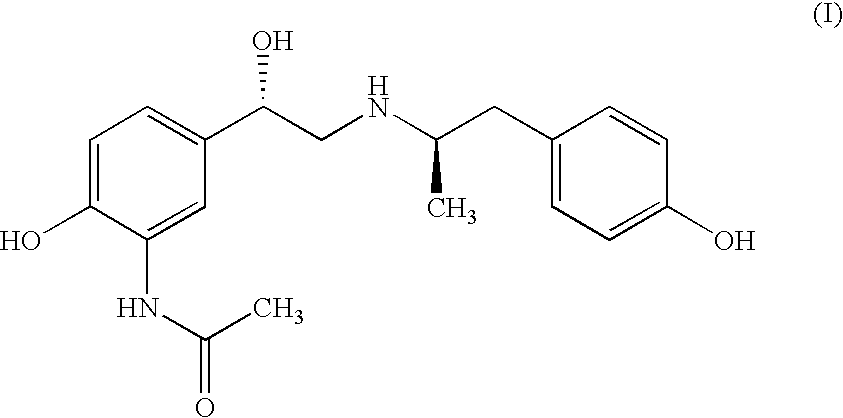
![Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid Inhalation particles comprising a salt of 8-hydroxy-2-[[(1R)-2-(4-methoxyphenyl)-1-methylethyl]amino] ethyl]-2(1H)-quinolinone and a corticosteroid](https://images-eureka.patsnap.com/patent_img/32424778-f83f-4807-814b-f6bddf6c9310/US20100269825A1-20101028-D00002.png)
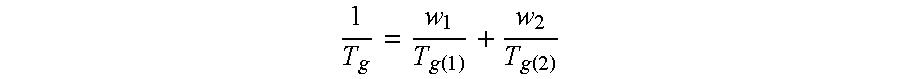
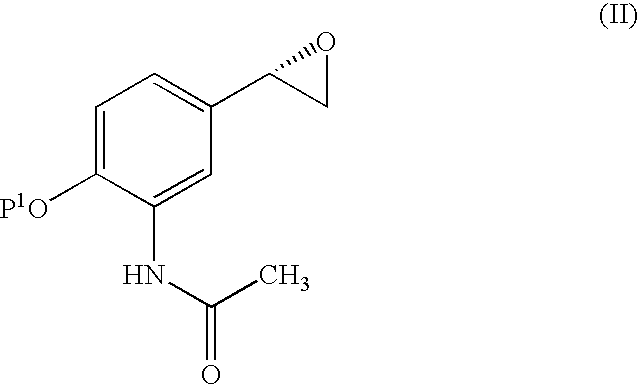
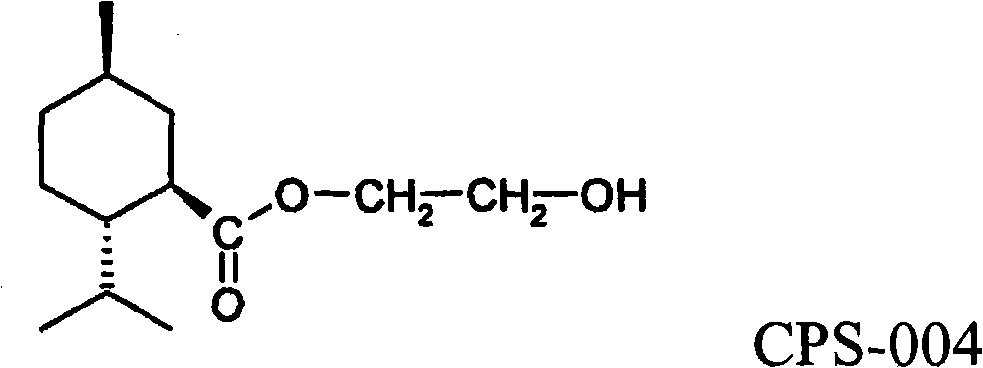
![Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors](https://images-eureka.patsnap.com/patent_img/c042cf2f-02a1-453d-8630-cd893ae71de0/US09573949-20170221-C00001.png)
![Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors](https://images-eureka.patsnap.com/patent_img/c042cf2f-02a1-453d-8630-cd893ae71de0/US09573949-20170221-C00002.png)
![Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors Derivatives of [1, 2, 4] triazolo [4, 3-a] pyridine as P38—MAP kinase inhibitors](https://images-eureka.patsnap.com/patent_img/c042cf2f-02a1-453d-8630-cd893ae71de0/US09573949-20170221-C00003.png)