Vaccine compositions
a technology of compositions and vaccines, applied in the field of vaccine compositions, can solve the problems of affecting the health system, affecting the treatment and prevention of allergic airways diseases, and the direct and indirect costs and achieve the effect of preventing or suppressing the onset of allergic airways diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Mouse Model of Th2-Induced Allergic Airways Diseases
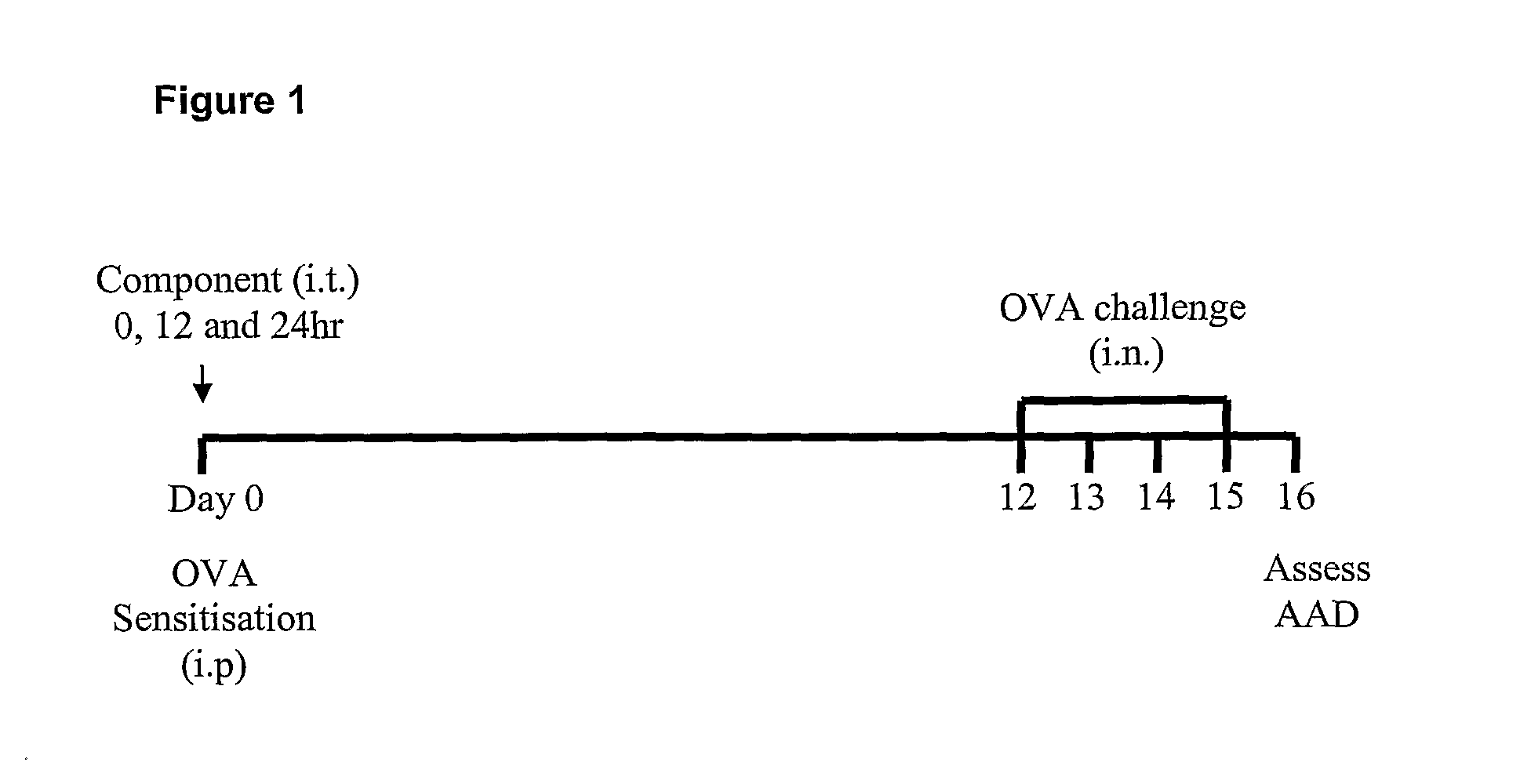
[0093]The present inventors have established an experimental model of allergic airways disease in BALB / c mice (Th2 and IgE responding), based on sensitization consistency and subsequent challenge with ovalbumin (OVA). This model is described in co-pending International PCT Application No. PCT / AU2007 / 001098, the disclosure of which is incorporated herein in its entirety.
[0094]Briefly, female BALB / c mice (6-8 week old) were obtained from the Central Animal House, University of Newcastle. For the induction of allergic airways disease, mice were sensitized by intraperitoneal injection of OVA (50 ug; Sigma, Missouri, USA) at day 0 using OVA in Rehydrogel (1 mg, Reheis, Berkeley Heights, USA) in sterile saline (200 μl) and mice were subsequently challenged by intranasal droplet application of OVA (day 12-15; 10 ug, 50 ul sterile saline).
[0095]For the experiments described in the subsequent examples, whole killed Streptococcus pneumoniae ...
example 2
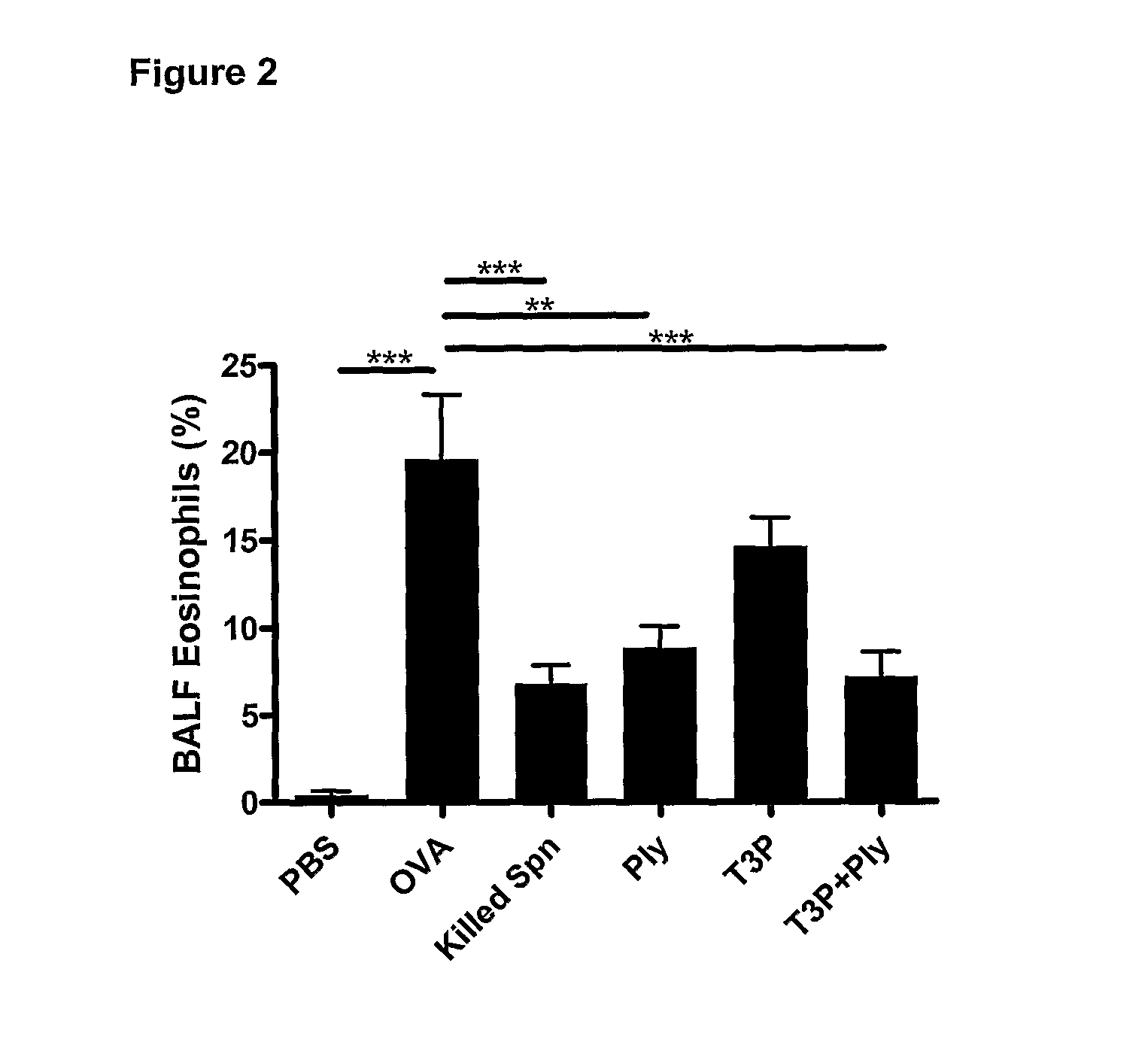
Effect of Vaccination Using Streptococcus pneumoniae Capsular Polysaccharide and Exotoxoid on the Development of Allergic Airways Diseases
[0102]Streptococcus pneumoniae (Type 3, strain NC012695; available under Accession Number ACTC6303 from the National Collection of Type Cultures, Egham, UK) was provided by Professor J. Kyd of the University of Can berra, Australia. Bacteria were cultured and prepared as described in Preston et al (2007). Briefly, prior to use, bacteria were stored at −80° C. in tryptone soya broth (TSB, Oxoid Australia) with 5% defibrinated horse blood, 0.5% glucose and 20% glycerol. Bacteria were freshly cultured before experiments on tryptone soy agar supplemented with 5% blood and 0.5% glucose and incubated for 16 hours (37° C., 5% CO2). Colonies were harvested, and suspended in phosphate buffered saline (PBS). Ethanol-killed Streptococcus pneumoniae were prepared and stored at −80° C. until required.
[0103]Pneumolysoid was obtained from Professor James Paton o...
example 3
Effect of Vaccination Using Streptococcus pneumoniae Capsular Polysaccharide, Exotoxoid, Cell Walls, CpG Oliguncleotides, and Combinations Thereof, on the Development of Allergic Airways Diseases
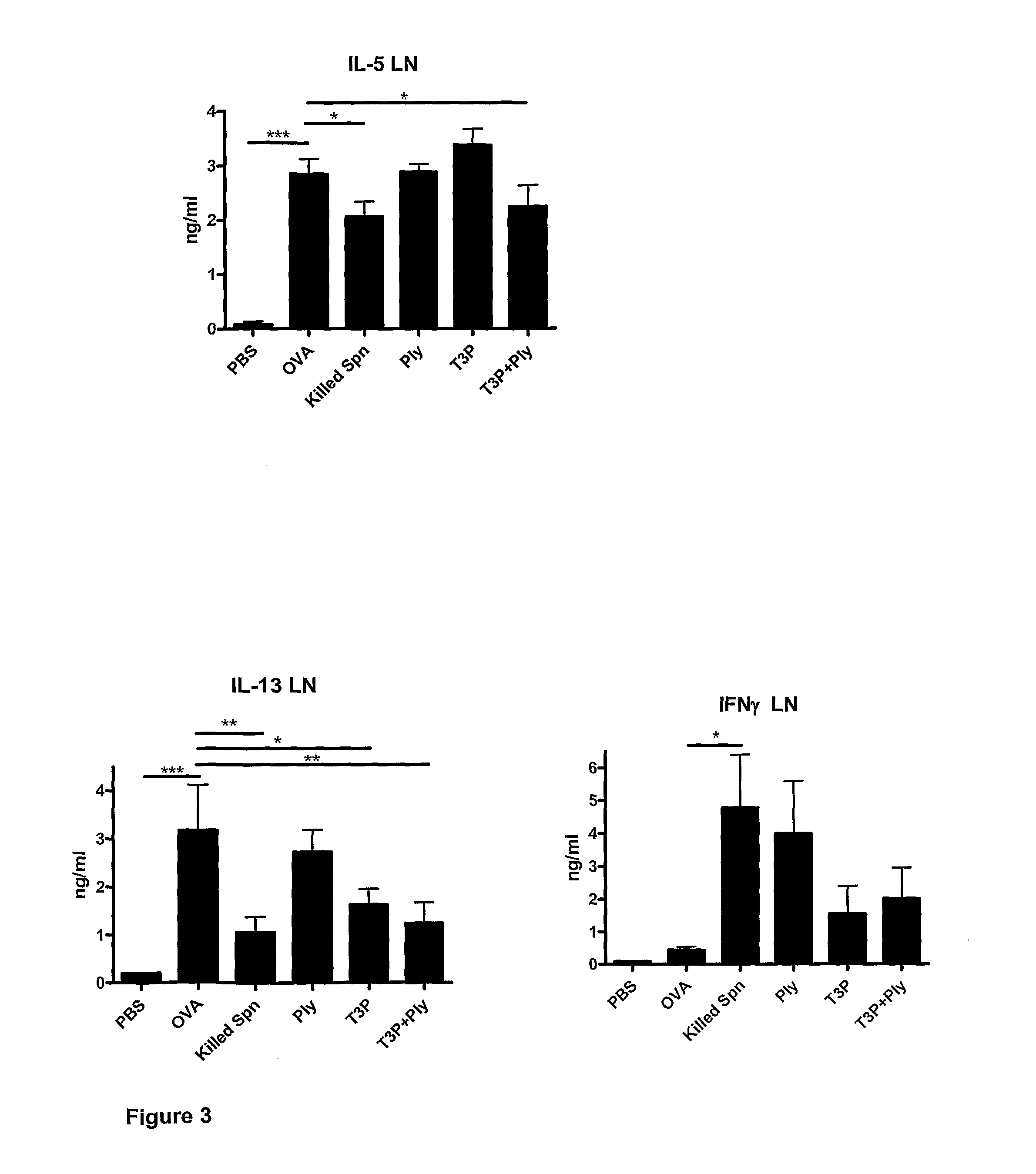
[0108]The inventors have also investigated the effect of the addition of purified cell walls from unencapsulated Streptococcus pneumoniae and of bacterial CpG oligonucleotides on BALF eosinophil levels and Th2 cytokine (IL-5 and IL-13) production. Four components (T3P, Ply, purified cell walls (CW) and CpG oligonucleotides (CpG)) were administered, alone, in combination, and in all possible pairwise and triplet combinations. Experiments were conducted as described above in Example 2.
[0109]Streptococcus pneumoniae cell walls were purified as described (Tuomanen et al., 1985) with minor modifications. Briefly, unencapsulated Streptococcus pneumoniae R6 (ATCC BAA-255) was cultured overnight in Heart Infusion (HI) media to 5×108 cfu / ml. Following centrifugation and supernatant removal, bacteria ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
| Strain point | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com