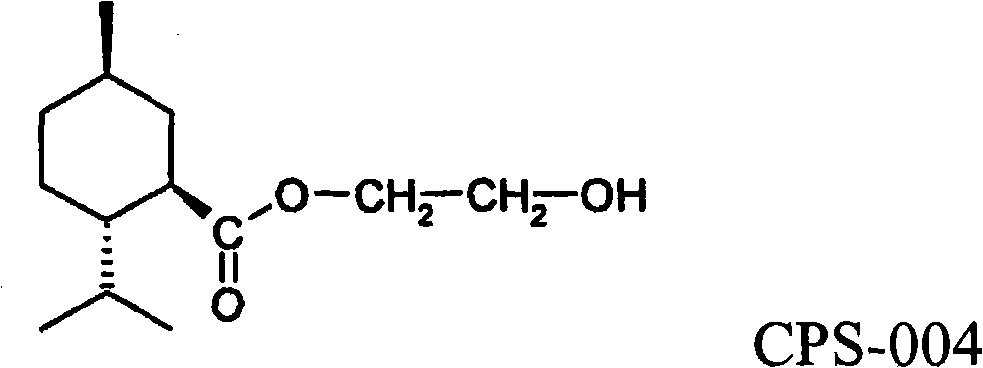P-menthawe-3-carboxylic acid esters to treat airways diseases
A technology for upper respiratory tract and diseases, which is applied in the field of p-menthane 3-carboxylate for the treatment of respiratory diseases, and can solve the problems of sugar and calorie increase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0171] Synthesis of hydroxy p-menthane carboxylate
[0172] (1R,2S,5R)-2-Isopropyl-5-methyl-2-hydroxyethyl cyclohexanecarboxylate (CPS-004)
[0173] (1R,2S,5R)-2-Isopropyl-5-methyl-cyclohexanecarboxylic acid 2,3-dihydroxypropyl ester (CPS-030)
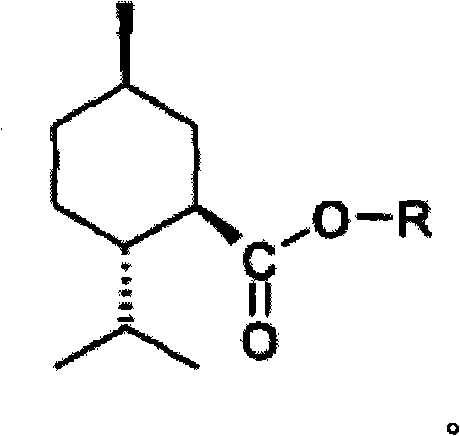
[0174]The synthesis of compounds of formula 1 is familiar to those skilled in the chemical arts and begins with the reaction of an appropriate alkyl-substituted cycloalkylcarboxylic acid with thionyl chloride to form the carbonyl chloride. The carbonyl chloride is then reacted with an excess of the appropriate alcohol derivative, optionally in the presence of a suitable hydrogen chloride acceptor or base. Many suitable alcohols are available from commercial sources such as Sigma-Aldrich Co., St. Louis, MO. A suitable method is described by Watson et al., 1977, US Patent 4,033,994. For example, for the synthesis of CPS-004, 5-methyl-2-methyl-2- Isopropylcyclohexanecarboxylic acid. Thus, the Grignard reagent is generated in dry tetr...
Embodiment 2
[0176] Octanol / water partition coefficient
[0177] The log P (octanol / water partition coefficient) of the compound of formula 1 was calculated and shown in the table. It can be seen that these compounds are compatible with the carrier for incorporation and can be stored in a depot for metered delivery using an aerosol dispenser.
[0178] Log P values are also given as parameters for the selection of primary supports and co-solvents. For example, CPS-160 has excellent water solubility with a low log P value, so it should be compatible with aqueous solvents containing 1% to 5% ethanol or propylene glycol. On the other hand, for long-term reduction of airway sensory irritation, such as in COPD, compound CPS-003 may be a better choice.
[0179]
[0180]
[0181] (*)CPS-004, (**)CPS-030
Embodiment 3
[0183] biometrics
[0184] CPS-004 and CPS-030 are liquids at room temperature. These compounds were tested by placing 0, 2, 5 and 8 mg of these compounds on a 6 inch glass rod and applying the compounds to the posterior 1 / 3 of the dorsal surface of the tongue. After depositing the test substance, the subject is instructed to keep the mouth closed and allow the test substance to distribute in the back of the mouth (oropharynx). The presence and duration of sensations from the oral cavity were then recorded.
[0185] Note that 2 mg of the substance has a cooling effect on the back of the mouth. CPS-004 and CPS-030 have similar potency. CPS-030 has a spicy taste, which is objectionable. The duration of the cooling sensation lasted 10 to 15 minutes, with higher doses producing a cooling sensation that was still perceptible after 30 minutes.
[0186] In the next set of experiments, CPS-004 or CPS-030 was mixed at 50 mg / ml in a solution of 5% ethanol-95% saline. 10 milliliter...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com