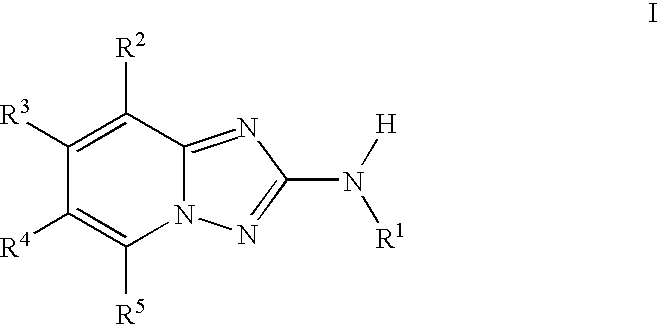
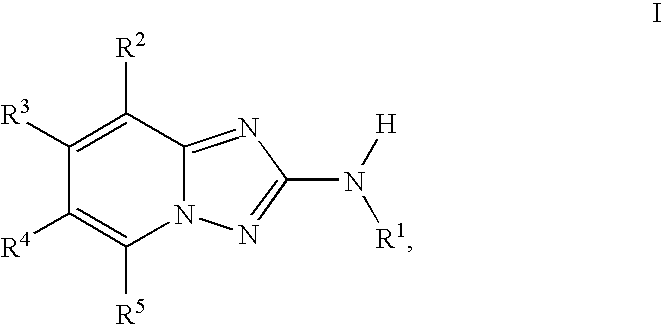
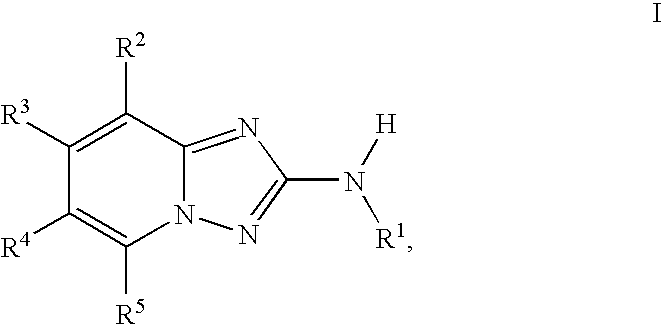
Triazolopyridine JAK Inhibitor Compounds and Methods
a technology of triazolopyridine and jak inhibitor, which is applied in the field of triazolopyridine jak inhibitor compounds and methods, can solve the problems of life-threatening thrombotic events and no known cure for ph mpd diseases, and achieve the effect of lessening the severity and lessening the severity of a diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example a
JAK2 Inhibition Assay Protocol
[0270]The activity of the isolated JAK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr) fluorescently labeled on the N-terminus with 5-carboxyfluorescein using the Caliper LabChip technology (Caliper Life Sciences, Hopkinton, Mass.). To determine the inhibition constants (Ki) of Examples 1-304, compounds were diluted serially in DMSO and added to 50 μL kinase reactions containing 0.2 nM purified JAK2 enzyme, 100 mM Hepes pH7.2, 0.015% Brij-35, 1.5 μM peptide substrate, 25 μM ATP, 10 mM MgCl2, 4 mM DTT at a final DMSO concentration of 2%. Reactions were incubated at 22° C. in 384-well polypropylene microtiter plates for 30 minutes and then stopped by addition of 25 μL an EDTA containing solution (100 mM Hepes pH 7.2, 0.015% Brij-35, 150 mM EDTA), resulting in a final EDTA concentration of 50 mM. After termination of the kinase reaction, the proportion of phosphoryla...
example b
JAK1 and TYK2 Inhibition Assay Protocol
[0271]The activity of the isolated JAK1 or TYK2 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Val-Ala-Leu-Val-Asp-Gly-Tyr-Phe-Arg-Leu-Thr-Thr) fluorescently labeled on the N-terminus with 5-carboxyfluorescein using the Caliper LabChip technology (Caliper Life Sciences, Hopkinton, Mass.). To determine the inhibition constants (Ki) of Examples 1-312, compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 1.5 nM JAK1, 0.2 nM purified JAK2 or 1 nM purified TYK2 enzyme, 100 mM Hepes pH7.2, 0.015% Brij-35, 1.5 uM peptide substrate, 25 uM ATP, 10 mM MgCl2, 4 mM DTT at a final DMSO concentration of 2%. Reactions were incubated at 22° C. in 384-well polypropylene microtiter plates for 30 minutes and then stopped by addition of 25 uL of an EDTA containing solution (100 mM Hepes pH 7.2, 0.015% Brij-35, 150 mM EDTA), resulting in a final EDTA concentration of 50 mM. After terminatio...
example c
JAK3 Inhibition Assay Protocol
[0272]The activity of the isolated JAK3 kinase domain was measured by monitoring phosphorylation of a peptide derived from JAK3 (Leu-Pro-Leu-Asp-Lys-Asp-Tyr-Tyr-Val-Val-Arg) fluorescently labeled on the N-terminus with 5-carboxyfluorescein using the Caliper LabChip technology (Caliper Life Sciences, Hopkinton, Mass.). To determine the inhibition constants (Ki) of Examples 1-312, compounds were diluted serially in DMSO and added to 50 uL kinase reactions containing 5 nM purified JAK3 enzyme, 100 mM Hepes pH7.2, 0.015% Brij-35, 1.5 uM peptide substrate, 5 uM ATP, 10 mM MgCl2, 4 mM DTT at a final DMSO concentration of 2%. Reactions were incubated at 22° C. in 384-well polypropylene microtiter plates for 30 minutes and then stopped by addition of 25 uL of an EDTA containing solution (100 mM Hepes pH 7.2, 0.015% Brij-35, 150 mM EDTA), resulting in a final EDTA concentration of 50 mM. After termination of the kinase reaction, the proportion of phosphorylated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com