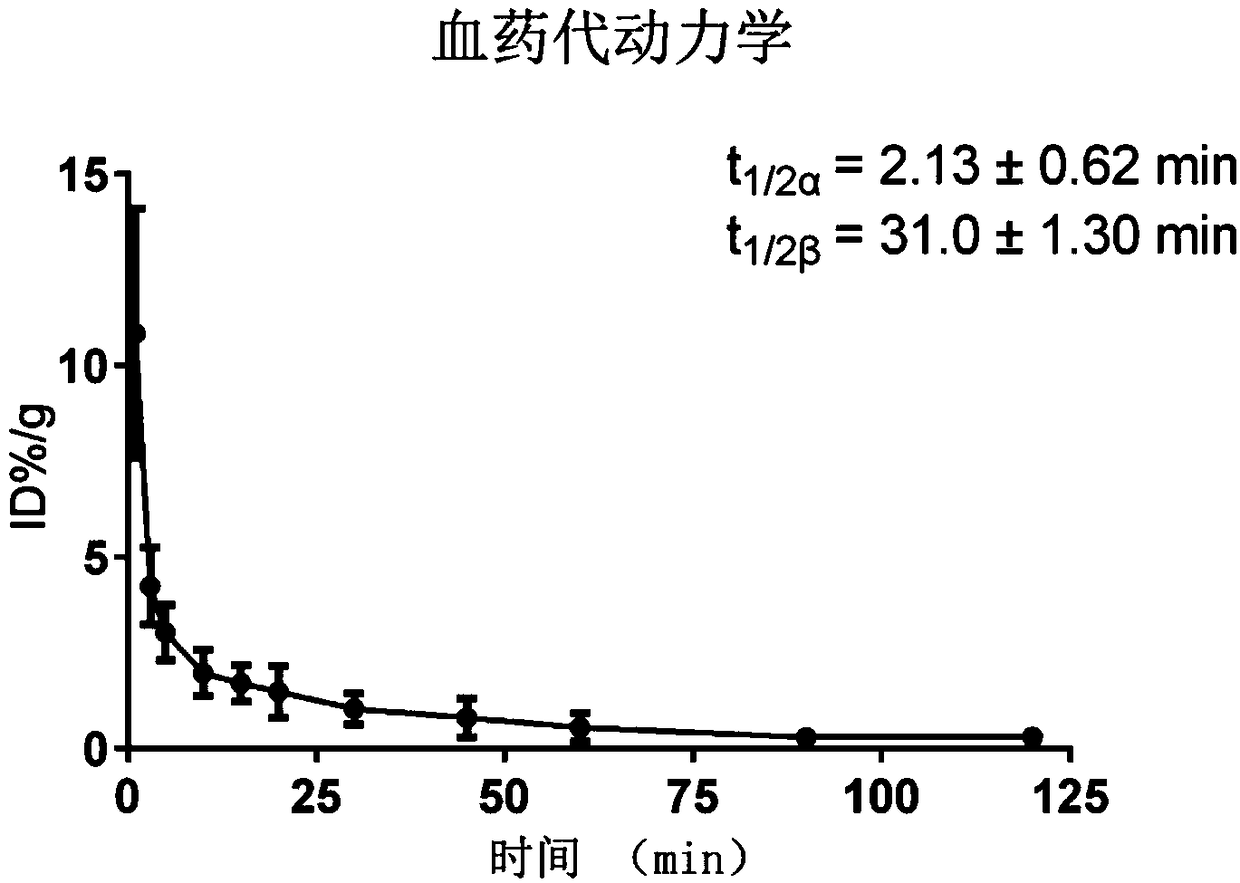
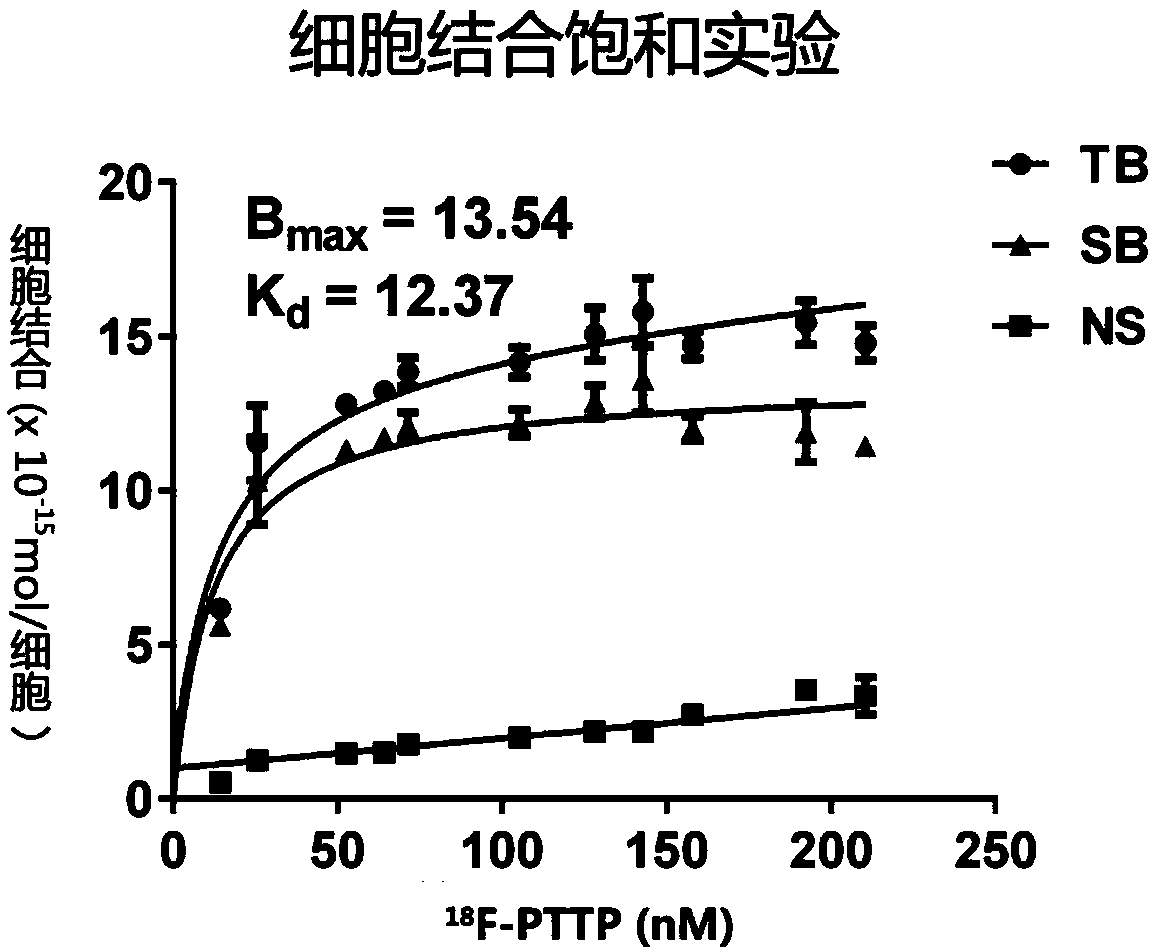
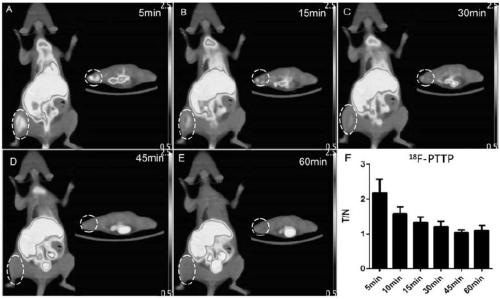
P2X7 receptor imaging agent and preparation method thereof
An imaging agent and receptor technology, applied in the field of F-18-labeled P2X7 receptor imaging agent and its preparation, can solve the problem of poor specificity of positron imaging agents, prone to false positives or false negatives, and inability to distinguish significantly problems such as tumors and inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthesis of embodiment 1 precursor pre-PTTP
[0040]
[0041] Add TEA (i.e. Et 3 N) (499.88 mg, 4.94 mmol), the mixture was stirred at 20° C. for 0.5 h, and the reaction was detected by LCMS to generate the target product (pre-PTTP). Add H to the reaction mixture 2 The reaction was quenched with O (100 mL), extracted with DCM (50 mL×3). The organic phases were combined, dried, and concentrated to obtain a crude product, which was separated by column chromatography (DCM:MeOH=1:0~10:1), and a white solid was obtained, which was the compound product (510.00 mg) shown in Formula II. rate 44%. The hydrogen spectrum and mass spectrum data of the precursor pre-PTTP are as follows:
[0042] 1 H NMR: ES6134-17-P1A (CDCl 3 ,400MHz)δ8.89-8.85(dd,J 1 =4.8Hz,J 2 =13.6Hz,2H),7.96-7.92(m,1H),7.43-7.39(m,1H),7.30-7.27(m,1H),7.07-7.04(m,1H),5.17-5.02(m,1H ),4.60-4.46(m,1H),4.23-4.07(m,1H),3.60-3.56(m,1H),3.44-3.43(m,1H),3.41-3.21(m,1H).
[0043] MS(ESI):467.0([M+H] + ...
Embodiment 2
[0044] Compound of Example 2 18 Synthesis of F-PTTP
[0045]
[0046] Before the synthesis reaction, the reaction raw materials were divided into bottle A and bottle B to prepare respectively.
[0047] Bottle B: methyl chlorodifluoroacetate ClCF 2 CO 2 Me (6 μL, 57 μmol), tetramethylethylenediamine TMEDA (10 μL, 63 μmol), precursor pre-PTTP (7 mg), ultra-dry DMF (500 μL, ensure absolute anhydrous).
[0048] Bottle A: Add CuI (15 mg, 78 μmol) and a stirrer in advance, and then dissolve the [ 18 F]KF / K 222 MeCN solution was added to the bottle, under N 2 Heating under gas protection to remove the solvent, then adding ultra-dry MeCN, under N 2 The solvent was removed by heating under gas protection, and so repeated three times, the H in the system 2 Remove all O, then add the solution in bottle B to bottle A, and react at 150°C for 20 minutes.
[0049] The reaction system was cooled to room temperature, and a small amount of water was added to quench the reaction. The...
Embodiment 318
[0050] Example 3 18 In vitro stability test of F-PTTP
[0051] With embodiment 2 gained respectively 18 About 10 μCi of F-PTTP were respectively placed in 100 μL of 0.9% normal saline and 0.1% BSA, mixed thoroughly and stored at 37°C. Samples were taken at 1h, 2h, 4h and 6h, and their purity changes were checked on analytical HPLC. The results are shown in Table 1.
[0052] Table 1. Compounds 18 HPLC detection results of F-PTTP in normal saline and BSA
[0053]
[0054] The results showed that the compound 18 F-PTTP is very stable, with only a small amount of decomposition within 6 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com