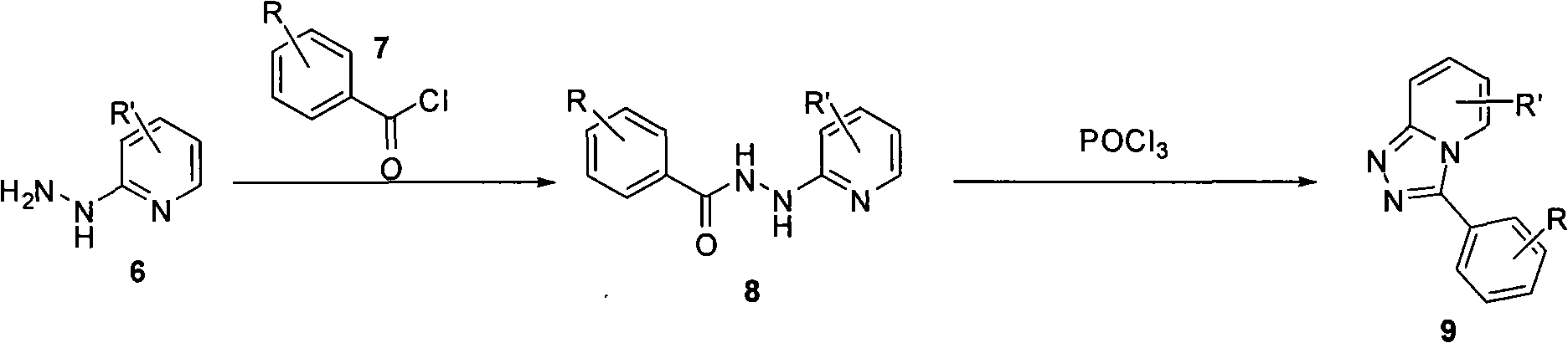
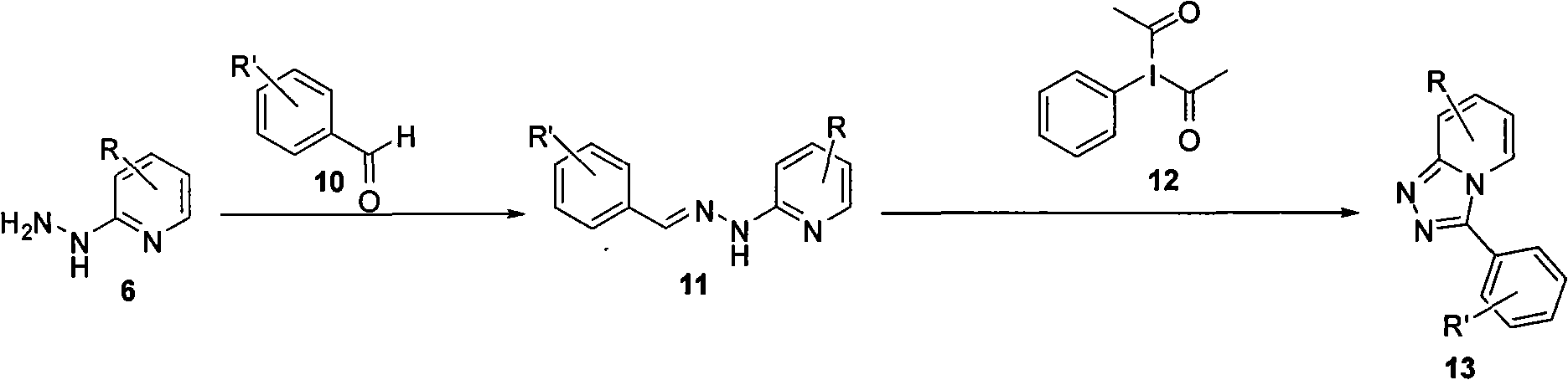
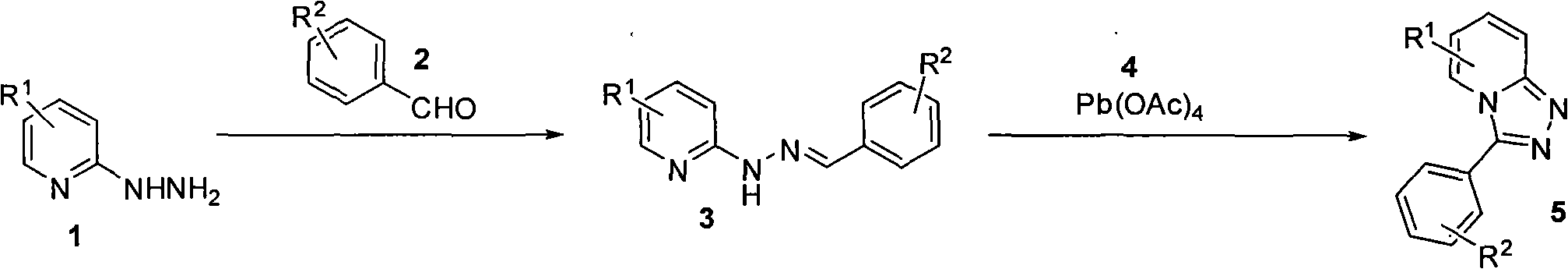
Synthesis method of triazol pyridine ring compound
A technology for cyclic compounds and azolopyridines, applied in the field of synthesis of important potential drug molecules triazolopyridine ring compounds, can solve the problems of low yield, difficult storage of raw materials, high cost of highly toxic raw materials, and achieves easy operation and post-processing. safe effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022]
[0023] method one:
[0024] The synthetic technique of 6-(trifluoromethyl)-3-m-tolyl-[1,2,4]triazol[4,3-a]pyridine:
[0025] Add 5 milliliters of absolute ethanol, 0.144 grams of m-tolualdehyde (2, 1.2 mmol), 0.177 grams of 2-hydrazino-5-trifluoromethylpyridine (1, 1.0 mmol) in a 25 milliliter single-necked bottle ) was heated to reflux for 2 hours, monitored by TLC, and after the aromatic aldehyde disappeared substantially, it was cooled to room temperature. The white solid pyridylhydrazone Schiff base formed in the reaction was filtered off. Dissolve it in 8 ml of anhydrous toluene, add 1.2 mmol of lead tetraacetate, and heat and stir at 80°C for 18 hours. After cooling to room temperature, 5 ml of water was added, and the precipitated solid was filtered and dissolved in ethyl acetate. The organic layer was washed with saturated sodium bicarbonate, brine, and dried over anhydrous sodium sulfate. Sodium sulfate was removed by filtration, the solvent was spun o...
Embodiment 2
[0032]
[0033] Replace m-methylbenzaldehyde in Example 1 with p-tolualdehyde to obtain 6-(trifluoromethyl)-3-p-tolyl-[1,2,4]triazol[4,3-a] Pyridine, the organic solvent is tetrahydrofuran, the reflux reaction time of the first step is 1 hour, and the reflux reaction time of the second step is 16 hours.
[0034] Yield: 78%. Its melting point: 108°C. H NMR spectrum 1 HNMR (CDCl 3 , 300MHz):: δ8.58(d, J=1.2Hz, 1H), 7.95(d, J=9.6Hz, 1H), 7.72(dd, J=1.8Hz, 6.6Hz, 2H), 7.46-7.38( m, 3H); mass spectrum (ESI source): C 14 h 10 f 3 N 3 m / z 278 (M + +1).
Embodiment 3
[0036]
[0037] Replace m-methylbenzaldehyde in Example 1 with o-methylbenzaldehyde to obtain 6-(trifluoromethyl)-3-o-tolyl-[1,2,4]triazol[4,3-a] Pyridine, the organic solvent is ethanol, the reflux reaction time of the first step is 1.5 hours, and the reflux reaction time of the second step is 20 hours.
[0038] Yield: 77%. Its melting point: 83°C. H NMR spectrum 1 HNMR (CDCl 3 , 300MHz):: δ8.16 (d, J = 1.2Hz, 1H), 7.98 (d, J = 9.6Hz, 1H), 7.56-7.40 (m, 5H); mass spectrum (ESI source): C 14 h 10 f 3 N 3 m / z 278 (M + +1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com