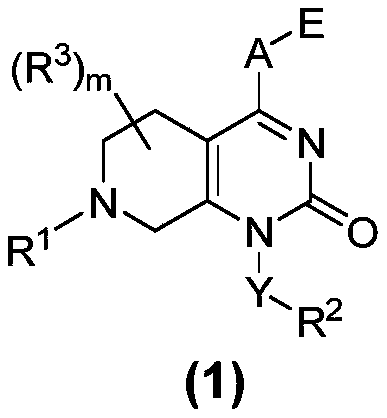
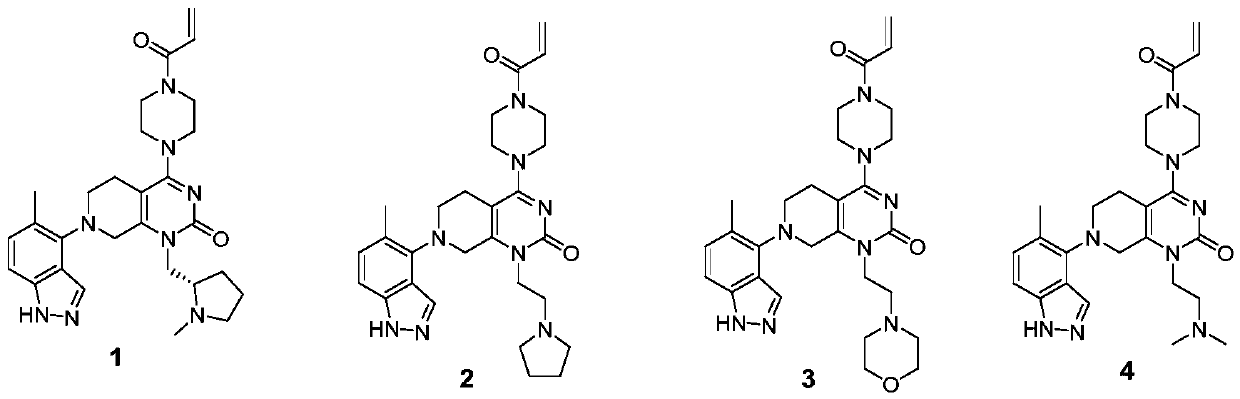
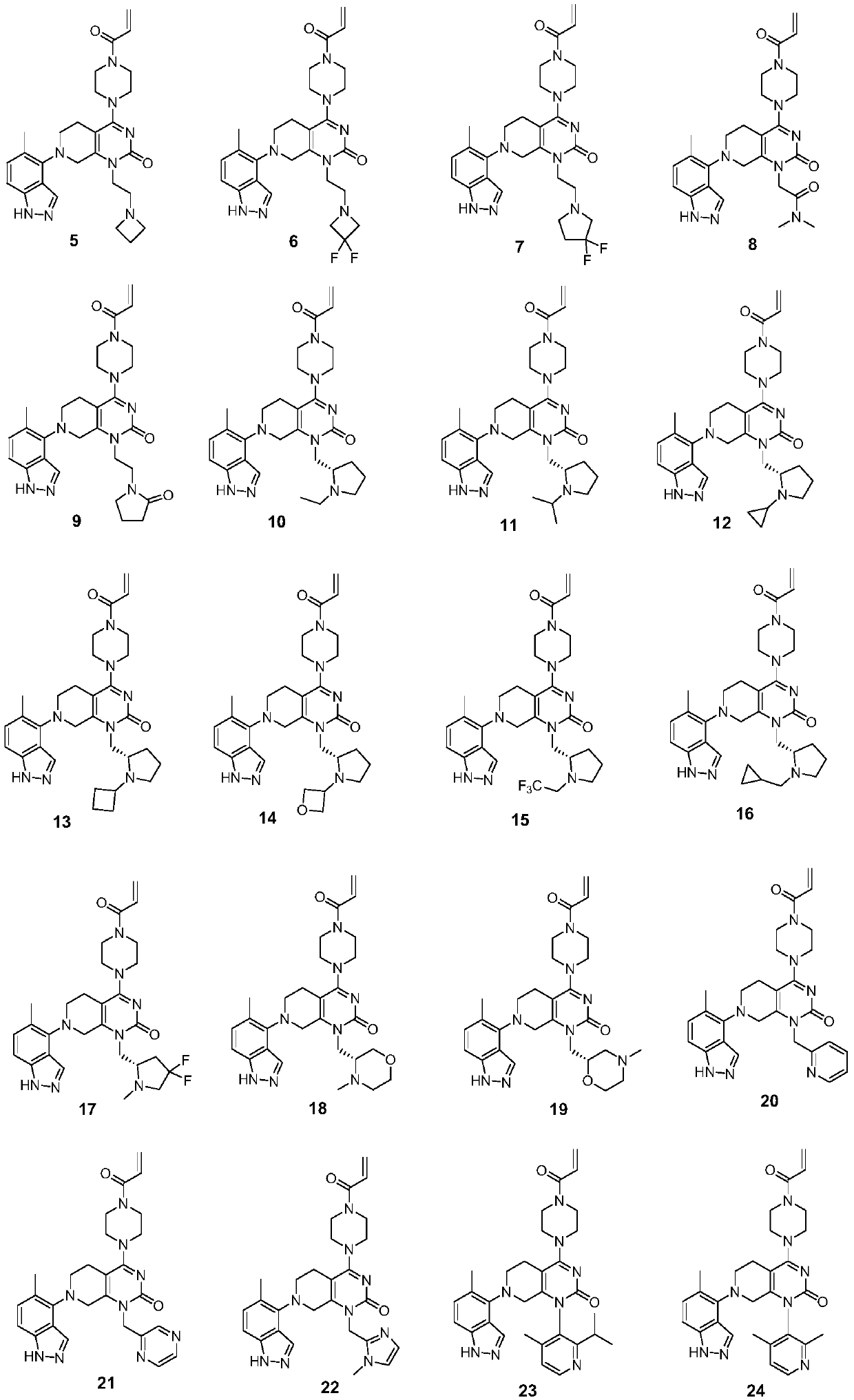
KRAS-G12C inhibitor
A C1-C3, C3-C6 technology, applied in the field of new KRAS-G12C inhibitors, can solve the problem of no anti-RAS therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] Example 1 (S)-4-(4-acryloylpiperazin-1-yl)-7-(5-methyl-1H-indazol-4-yl)-1-((1-methylpyrrolidine Synthesis of -2-yl)methyl)-5,6,7,8-tetrahydropyrido[3,4-d]pyrimidin-2(1H)-one (Compound 1)
[0147]
[0148] Compound 1 was prepared according to Method A as described below:
[0149] 4-(4-((Benzyloxy)carbonyl)piperazin-1-yl)-2-(methylmercapto)-5,8-dihydropyrido[3,4-d]pyrimidine-7(6H)- tert-butyl formate (1-1)
[0150] A1 (5.2g, 12.11mmol), DIPEA (3.2g, 24.22mmol), benzyl-1-piperazine carbonate (2.9g, 13.31mmol) and DMF (50mL) were added to a 250mL single-necked bottle, under the protection of Ar, Raise the temperature to 100°C for 1 hour reaction. TLC monitoring (PE / EA=10 / 1), the raw materials reacted completely, after cooling the reaction solution to room temperature, added water (100mL), extracted with EA (50mL*2), combined the organic phases, washed with saturated sodium chloride, organic The phase was concentrated and purified by column chromatography (PE / EA=1 / 0 to ...
Embodiment 2
[0168] Example 2 4-(4-acryloylpiperazin-1-yl)-6-cyclopropyl-8-methoxy-7-(5-methyl-1H-indazol-4-yl)-1- Synthesis of (((S)-1-methylpyrrolidin-2-yl)methyl)quinazolin-2(1H)-one (Compound 53)
[0169]
[0170] Compound 53 was prepared according to Method B as described below:
[0171] tert-butyl 4-(7-bromo-2,6-dichloro-8-fluoroquinazolin-4-yl)piperazine-1-carboxylate (53-1)
[0172] Add B1 (5g, 15.13mmol), DIPEA (4g, 30.3mmol), N-Boc-piperazine (2.96g, 15.89mmol) and DMF (100mL) into a 250mL single-necked bottle, under the protection of Ar, heat up to 80°C for reaction 2 Hour. TLC spot plate (PE / EA=5 / 1), the reaction of raw materials was complete, after cooling the reaction solution to room temperature, add water (100mL), extract with EA (100mL*2), combine the organic phases, wash with saturated sodium chloride, The organic phase was concentrated and purified by column chromatography (PE / EA=1:0 to 2 / 1) to give white solid 53-1 (5.23g, yield 72%), ESI-MS m / z: 479.1 / 481.1[M+ H...
Embodiment 3
[0188] Example 3 4-((S)-4-acryloyl-2-methylpiperazin-1-yl)-6-fluoro-7-(2-fluoro-6-hydroxyphenyl)-1-((( Synthesis of S)-1-methylpyrrolidin-2-yl)methyl)pyrido[2,3-d]pyrimidin-2(1H)-one (Compound 74)
[0189]
[0190] Compound 74 was prepared according to Method C as described below:
[0191] 2,6-Dichloro-5-fluoronicotinamide (74-1)
[0192] CDI (35.6g, 220mmol) was added in batches to a solution of C1 (42g, 200mmol) in THF (400mL), the mixture was stirred for 5min, protected by Ar, heated to 50°C, reacted for 1h, monitored by LC-MS, the raw material disappeared, and the reaction The solution was diluted with toluene (100 mL), concentrated to half of the original volume, the resulting mixture was cooled to 0 °C, and ammonium hydroxide (55 mL, 400 mmol) was added slowly. React at room temperature for 10 minutes, dilute with EA (200mL), and wash with water (100mL*3). Anhydrous Na for organic layer 2 SO 4 To dry, spin dry. PE / EA (10 / 1,200 mL) was beaten, filtered, the remai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com