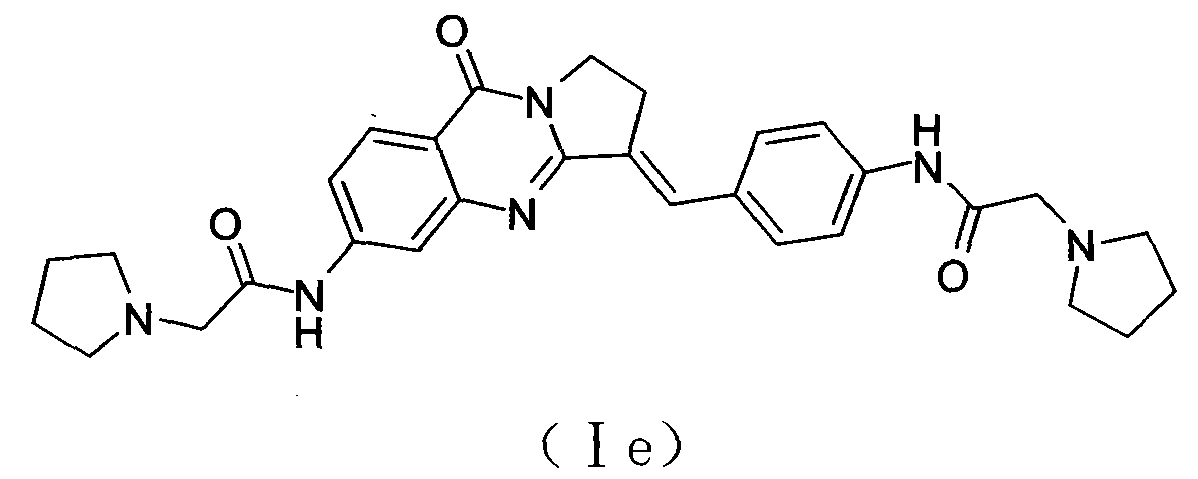
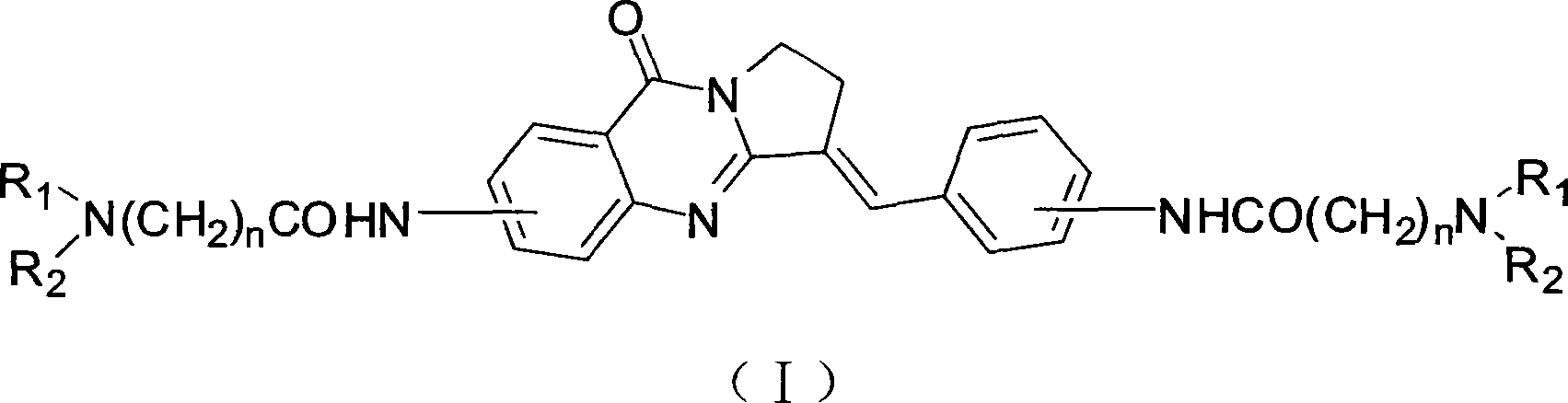
Bisfatty amido substituted quinazolone derivatives as well as preparation method and use thereof as anti-cancer drugs
A technology of quinazolones and derivatives, which is applied in the field of new drug compounds, can solve problems such as reports on the anti-cancer effects of di-fatty amino-substituted quinazolones derivatives, etc., and achieve cheap raw materials, simple preparation methods, and low cost. Toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound 2
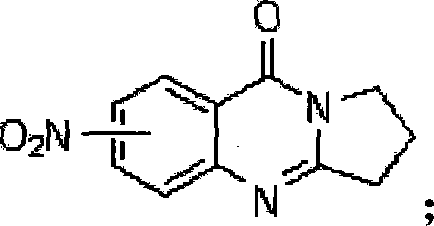
[0029] Dissolve 0.01mol of dry 4-nitro-2-aminobenzoic acid and 0.005mol of pyrrolidone in 250ml of toluene, add 2ml of phosphorus oxychloride dropwise at room temperature, raise the temperature to 110°C and react at this temperature for 5-12 hours . The reaction solution was slowly poured into ice water, adjusted to pH = 7, and suction filtered to obtain a yellow solid powder. The crude product was recrystallized with acetone to obtain a light yellow solid powder, namely compound 2, whose chemical formula is shown in formula (IIa):
[0030]
[0031] Yield: 60%; 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 2.30-2.40 (m, 2H); 3.23 (t, J = 7.8Hz, 2H); 4.24 (t, J = 7.2Hz, 2H); 8.17 (dd, J = 8.7, 2.1Hz, 1H); 8.40(d, J=8.7Hz, 1H); 8.45(d, J=2.1Hz, 1H); ESI-MS m / z: 232[M+H] + .
Embodiment 2
[0032] The synthesis of embodiment 2 compound 2
[0033] The method is the same as in Example 1, except that 5-nitro-2-aminobenzoic acid is used instead of 4-nitro-2-aminobenzoic acid to obtain a light yellow solid that is compound 2, and its chemical formula is as shown in formula (IIb) :
[0034]
[0035] Yield: 65%; 1 H-NMR (300MHz, CDCl 3 )δ (ppm): 2.29-2.40 (m, 2H); 3.23 (t, J = 7.8Hz, 2H); 4.24 (t, J = 7.2Hz, 2H); 7.72 (d, J = 8.7Hz, 1H) ; 8.48 (dd, J = 8.7, 2.7Hz, 1H); 9.11 (d, J = 2.7Hz, 1H); ESI-MS m / z: 232 [M+H] + .
Embodiment 3
[0036] The synthesis of embodiment 3 compound 3
[0037] In molar ratio, dissolve 0.001 mol of compound 2 prepared in Example 1 and 8 to 10 times the amount of 4-nitrobenzaldehyde in 10 ml of acetic anhydride, reflux for 24 to 32 hours, cool to room temperature, suction filter, and use for solid Wash with ethanol, chloroform, and acetone several times. Dissolve the obtained intermediate product in 10ml of ethanol and heat to nearly reflux, add 16ml of aqueous solution dissolved with 0.004mol sodium sulfide nonahydrate and 0.01mol sodium hydroxide, reflux for 4-8 hours, cool to room temperature, and stand overnight. Ethanol was removed by rotary evaporation, and then cooled at 0-5°C, filtered with suction, washed with water several times to obtain a dark red crude product. The crude product was recrystallized with ethanol / acetone to obtain an orange-red solid, namely compound 3, whose chemical formula is shown in formula (IIIa):
[0038]
[0039] Yield: 60%; 1 H-NMR (500M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com