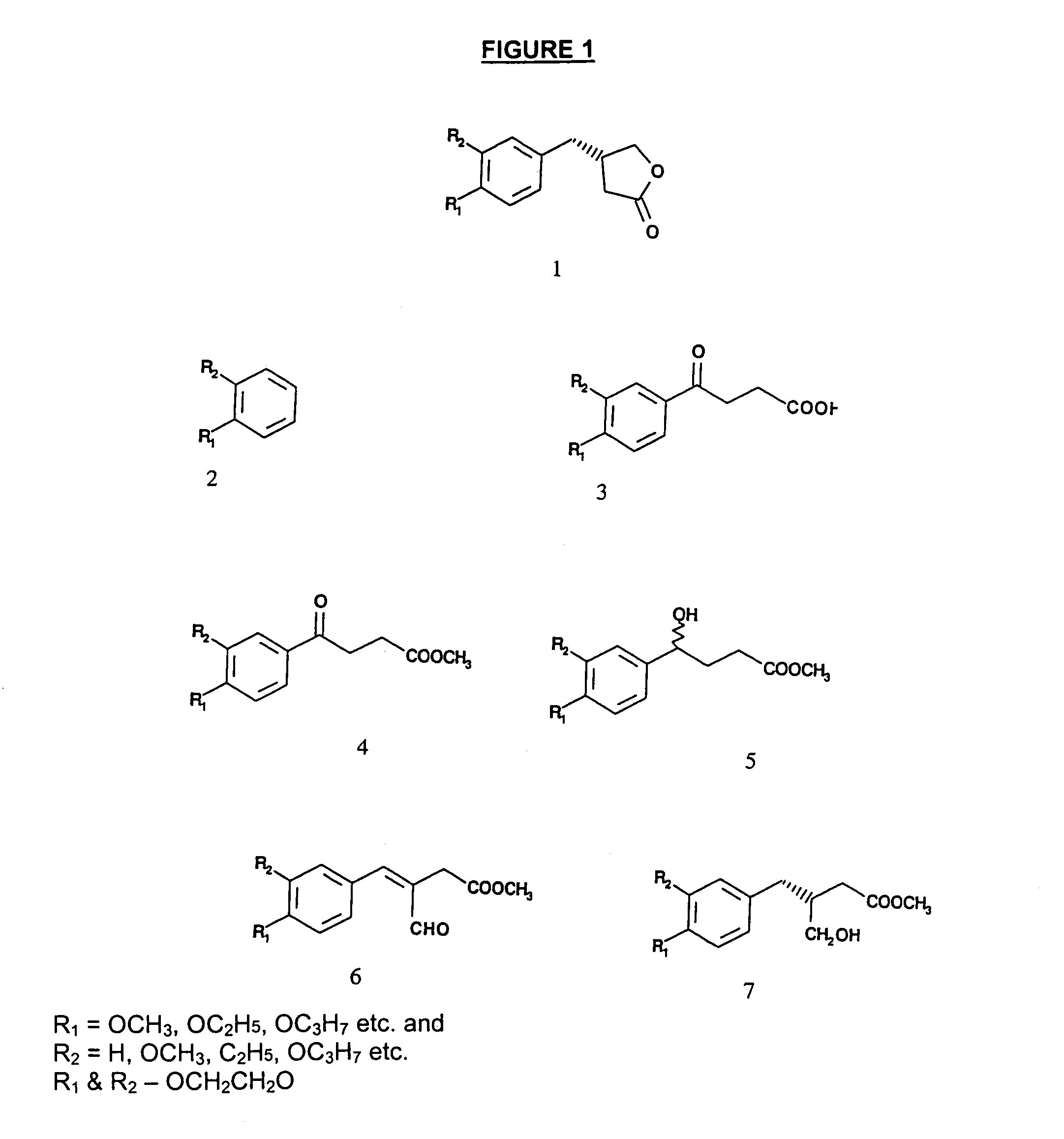
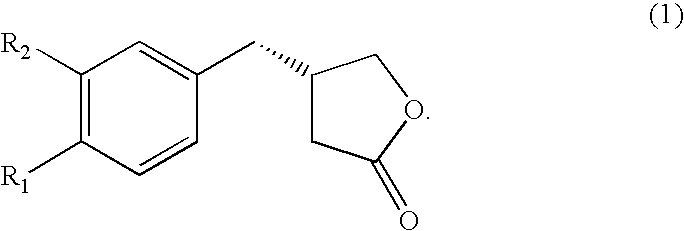
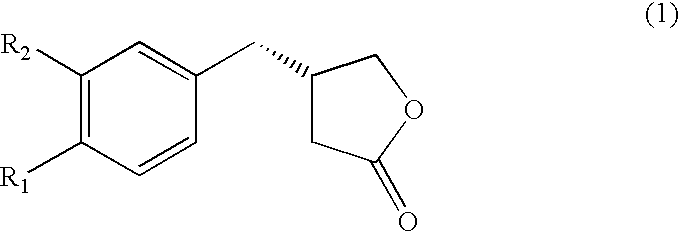
Chemo-enzymatic process for the preparation of opticaly enriched beta-benzyl-gamma-butyrolactones
a technology of beta-benzylgammabutyrolactone and chemoenzymatic process, which is applied in the field of new products, can solve the problems of complex experimental conditions, low over all yield, and inconvenient higher-scale synthesis of known processes or substituted -benzyl--butyrolactones
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example-1
Step-1
Preparation of 4-(3,4-dimethoxyphenyl)-4-oxo-butyric acid (3) R1═R2═OCH3)
[0039]In a three necked flask fitted with a guard tube, a mixture of veratrole (3,4-dimethoxy benzene, 2) (7.0 g, 12H14O5 (found C, 61.44%, H, 5.88% requires C60.49%; H 5.92%).
[0040]1H NMR (CD3OD): 2.67 (2H, t, J=6.6 Hz, H-2), 3.27(2H, t, J=6.6 Hz, H-3), 3.86 & 3.89(6H, 2xs, 2xOCH3), 7.02(1H, d, J=8.5 Hz, Ar—H), 7.65(1H, d, J=2.0 Hz, Ar—H), 7.68(1H, dd, J=8.5 & 2.0 Hz, Ar—H).
[0041]IR (KBr): 3382, 2940, 1730, 1660, 1592, 1504, 1444, 1414, 1332, 1264, 1240, 1140, 1018, 874 cm−1.
Step-2
Preparation of methyl[4-(3,4-dimethoxyphenyl)-4]-oxo-butyrate (4) R1═R2═OCH3)
[0042]The 4-(3,4-dimethoxyphenyl)-4-oxo-butyric acid (5 g.) in diethyl ether is esterified with a freshly prepared ethereal solution of diazomethane to furnish the corresponding ester in quantitative yield, which on crystallization from ethyl acetate / n-hexane gave colorless needles of 4 mp 87° C., analyzed for C13H16O5 (found C 62.14, H 6.41 requires C...
example-2
Step-1
Preparation of 4-(4-methoxyphenyl)-4-oxo-butyric acid (3) (R1 and R2═OCH3 and (H)
[0059]The title compound was prepared from anisole (11 g, 102 mmol) and succinic anhydride (i2 g, 120 m mol) in presence of anhydrous aluminum chloride (30 g) by the same method as described for 2 above to furnish 4-(4-methoxyphenyl)-4-oxo-butanoic acid in 84.6% yield, which on purification and crystallization from methanol / ethyl acetate (1:9) produced white crystals of 3, mp142–43° C., analyzed for C11,H12, O4 (found C64.21, H5.86; requires C63.45, H5.80%).
[0060]1H NMR (CDCl3-+DMSO-d6): 2.70 (2H, t, J=6.5 Hz, H-2), 3.26 (2H, t, J=6.5 Hz, H-3), 3.93 (3H, s, OCH3), 7.04 (2H, d,J=8.5 Hz, Ar—H), 8.05 (2H, d, J=8.5, Ar—H).
[0061]13CNMR (CDCl3+DMSO-d6): 28.1, 32.9, 55.4, 114.2, 129.7, 130.1, 162.7, 174.6, 196.7.
[0062]IR (KBr): 2844, 1670, 1600, 1574, 1426, 1358, 1316, 1246, 2120, 1026, 930, 830 cm−1.
[0063]M+ at m / z, 208 (40), 135 (83), 120 (12), 106 (69), 91(100), 77 (100).
Step-2
Preparation of methyl[4-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com