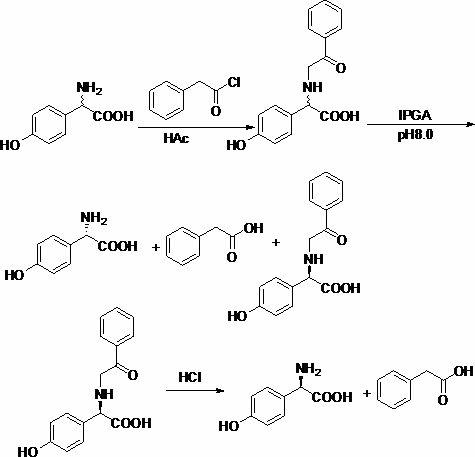
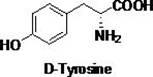
Chemical-enzyme method for preparing D-tyrosine
A tyrosine and chemical technology, applied in the field of chemical enzymatic method for preparing D-tyrosine, can solve the problems of difficult removal of L-tartaric acid, low optical purity, etc., and achieve reduced process cost, high optical activity, and simplified enzyme The effect of catalytic reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Preparation of DL-N-phenylacetyltyrosine
[0019] Add 72.4g (0.4mol) DL-tyrosine and 48g acetic acid (0.8mol) into 800ml water, stir to dissolve. 64ml (0.48mol) of phenylacetyl chloride was added dropwise under ice bath conditions. After the dropwise addition was completed, the reaction was carried out overnight at 60°C. Cool in an ice bath to 5°C, filter with suction, wash the solid with a small amount of deionized water, and dry to obtain 110.2 g of DL-N-phenylacetyltyrosine, with a yield of 92.1%.
Embodiment 2
[0020] Example 2: DL-N-phenylacetyltyrosinase catalyzed hydrolysis
[0021] Add 89.7g (0.3mol) of DL-N-phenylacetyltyrosine to 1000ml of water, and adjust the pH to 8.0 with ammonia water. 17.9 g of immobilized penicillin acylase was added, and the reaction was stirred at 30° C. for 10 h. Suction filtration to remove immobilized penicillin acylase. The filtrate was adjusted to pH 1-2 with concentrated hydrochloric acid, filtered with suction, the solid was washed with cold water, and dried to obtain 40.4 g of D-N-phenylacetyltyrosine with a yield of 45%.
Embodiment 3
[0022] Embodiment 3: Preparation of D-tyrosine
[0023] Dissolve 40.4g of D-N-phenylacetyltyrosine in 200mL of 6 mol / L concentrated hydrochloric acid, N 2 protection, hydrolysis at 90°C for 12 hours, adding 10 g of activated carbon to the hydrolyzate for decolorization. Concentrate the decolorized solution to 40 ml, extract three times with ethyl acetate (40ml×3), adjust the pH of the aqueous layer to 5.6 with ammonia water, let stand overnight at 0-5°C, filter and wash the filter cake with a small amount of deionized water, and dry it 22.5 g of D-tyrosine was obtained, the yield was 92%, and the ee measured by HPLC was 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com