Process for the preparation of simvastatin
A technology of simvastatin and separation method, which is applied in the field of preparation of simvastatin, can solve the problems of many reaction steps, high energy consumption, low output rate, etc., and achieve the effect of simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
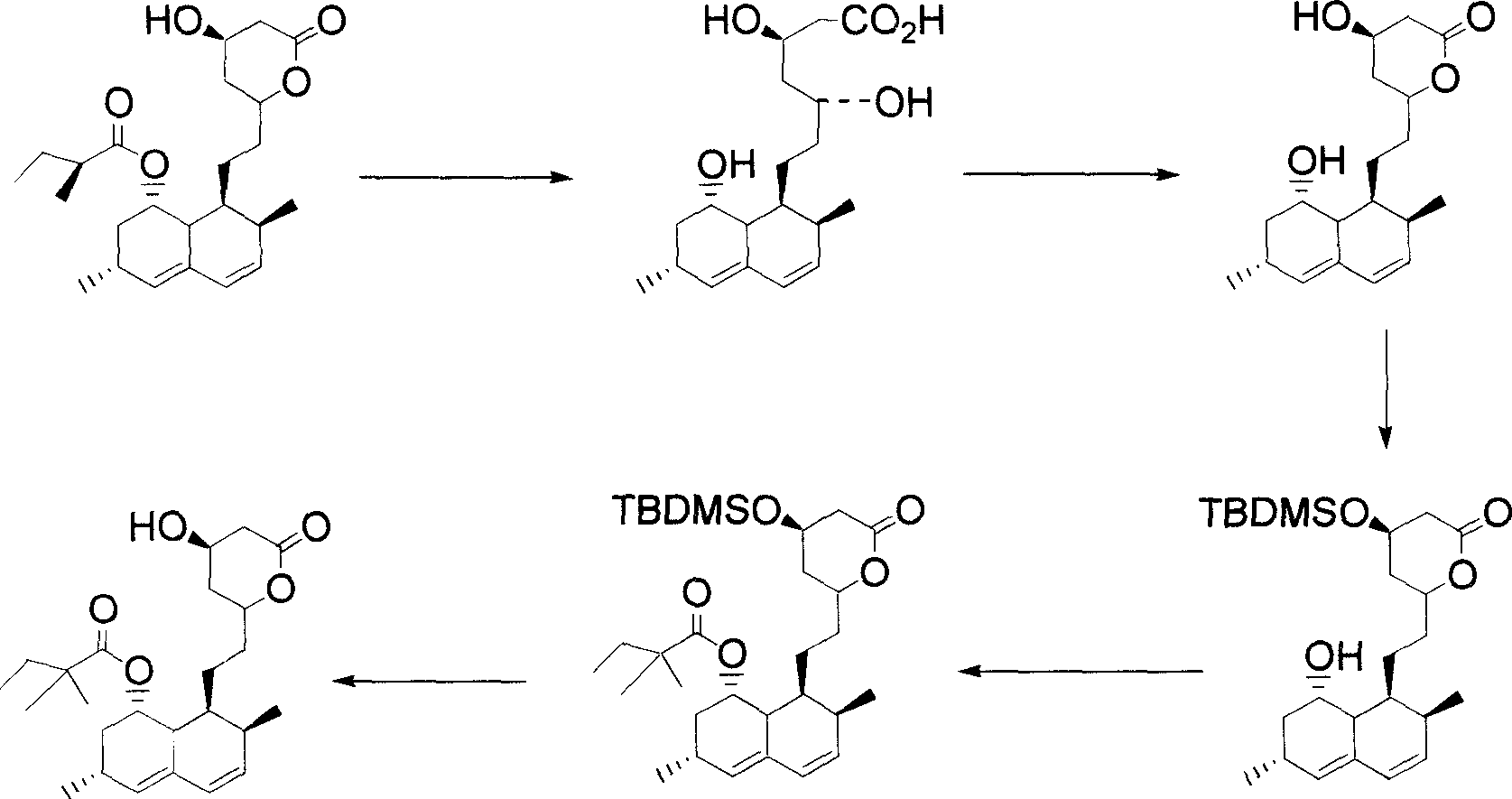
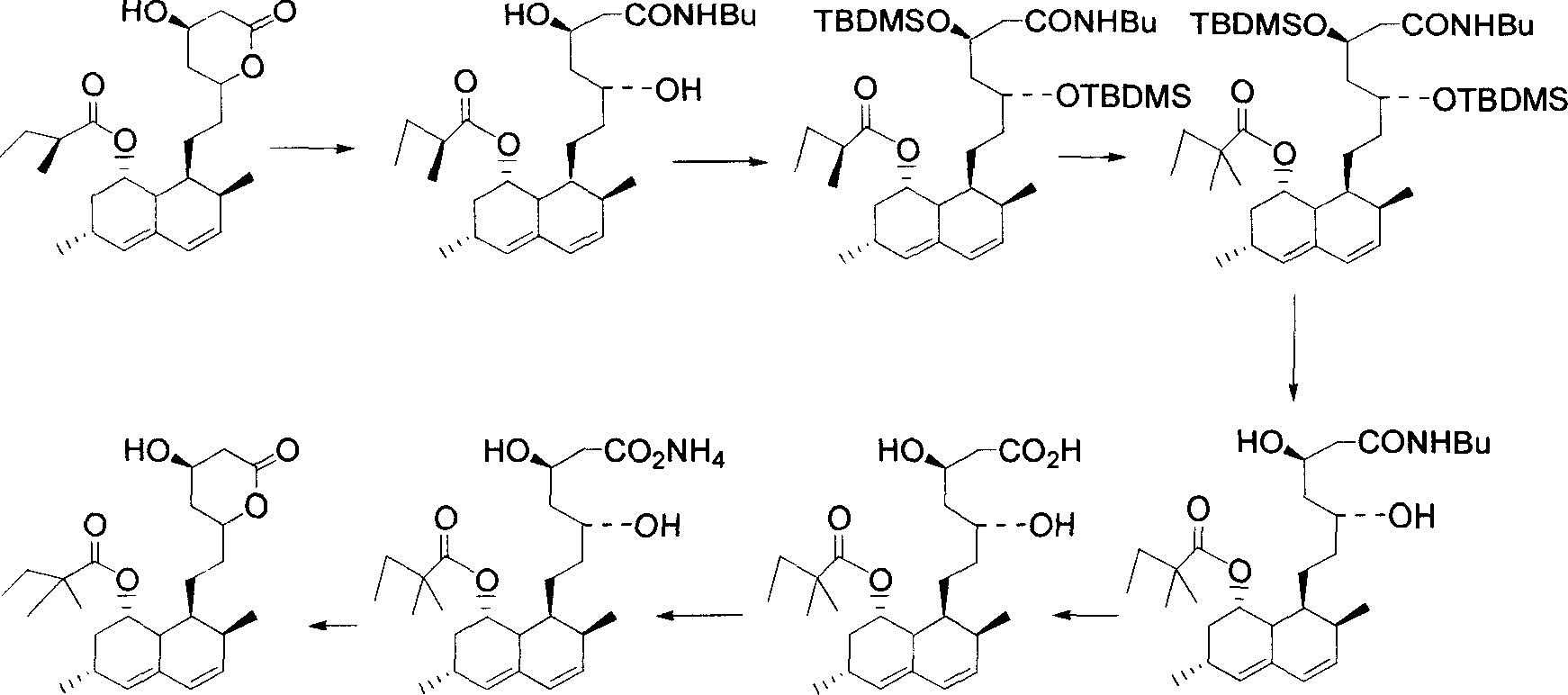
[0069] The hydrolysis of embodiment one lovastatin and the generation of trihydroxy acid molecular intermediate (reaction 4)
[0070] Under nitrogen protection, 20.0 g of lovastatin was dissolved in 200 ml of hot ethanol. At room temperature, 100 ml of cold potassium hydroxide (36 g) aqueous solution was slowly added to the above reaction solution. The reaction mixture was stirred at room temperature under nitrogen protection for 0.5 to 1 hour, and then refluxed for 12 to 16 hours. Then add 300 ml of water, evaporate 500 ml of solvent, and cool to 5-10°C. Then add 80 ml of diethyl ether, slowly add concentrated hydrochloric acid to adjust the pH value to 5.0 and control the temperature between 5-10°C during the process of adding concentrated hydrochloric acid. Stirring was kept for another hour, and the trihydroxy acid molecular intermediate was crystallized and precipitated in ether, and the obtained solid product was washed with water and dried under vacuum.
Embodiment 2
[0071] The synthesis (reaction 5) of the simvastatin derivative of embodiment two formula (4)
[0072] Under nitrogen protection, 16.0 g of the dried trihydroxy acid molecular intermediate was suspended in 300 ml of dichloromethane. After adding 0.4 g of p-toluenesulfonic acid, heat back distillation and distill off about 100 ml of dichloromethane. The white solid disappeared quickly and dissolved to give a clear solution. After cooling to a temperature of 5-10° C., 0.5 molar equivalents of lithium bromide, 2.1 molar equivalents of triethylammonia, and 2.4 molar equivalents of 2,2-dimethyl-butyryl chloride were added. After the reaction mixture was stirred under nitrogen for 0.5 to 1 hour, the reaction was stirred at room temperature. After the reaction was completed, 100 ml of water was added, and the organic layer was separated after stirring for 30 minutes. The organic layer was washed once with saturated brine (100 ml), washed four times with saturated aqueous sodium bi...
Embodiment 3
[0073] The synthesis (reaction 5) of the simvastatin derivative of embodiment three formula (4)
[0074] Under nitrogen protection, 16.0 g of the dried trihydroxy acid molecular intermediate was suspended in 300 ml of dichloromethane. After adding 0.4 g of p-toluenesulfonic acid, heat back distillation and distill off about 100 ml of dichloromethane. The white solid disappeared quickly and dissolved to give a clear solution. Then cool to a temperature of 5-10° C., and then add 0.5 molar equivalents of lithium bromide, 2.1 molar equivalents of triethylammonia, and 2.4 molar equivalents of 2,2-dimethyl-butyryl anhydride. After the reaction mixture was stirred under nitrogen for 0.5 to 1 hour, the reaction was stirred at room temperature. After the reaction was completed, 100 ml of water was added, and the organic layer was separated after stirring for 30 minutes. The organic layer was washed once with saturated brine (100 ml), washed four times with saturated aqueous sodium b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com