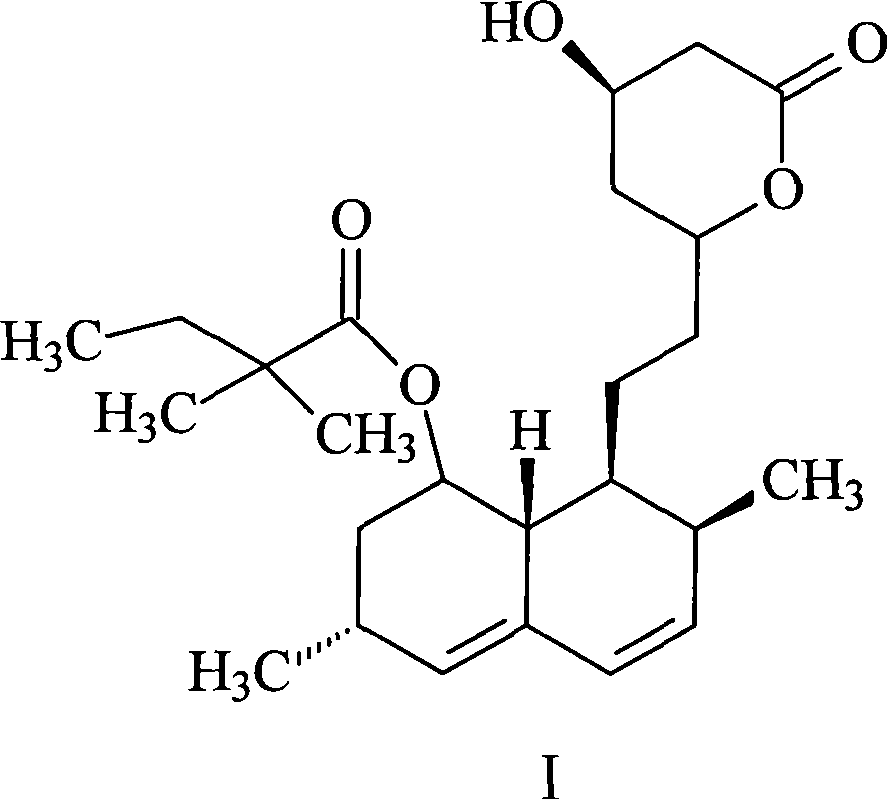
Preparation method of simvastatin
A technology of simvastatin and lovastatin amide, which is applied in the field of preparation of simvastatin, can solve the problems of short process route, high price, long process route, etc., and achieve the effect of readily available raw materials and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of this simvastatin comprises the steps:
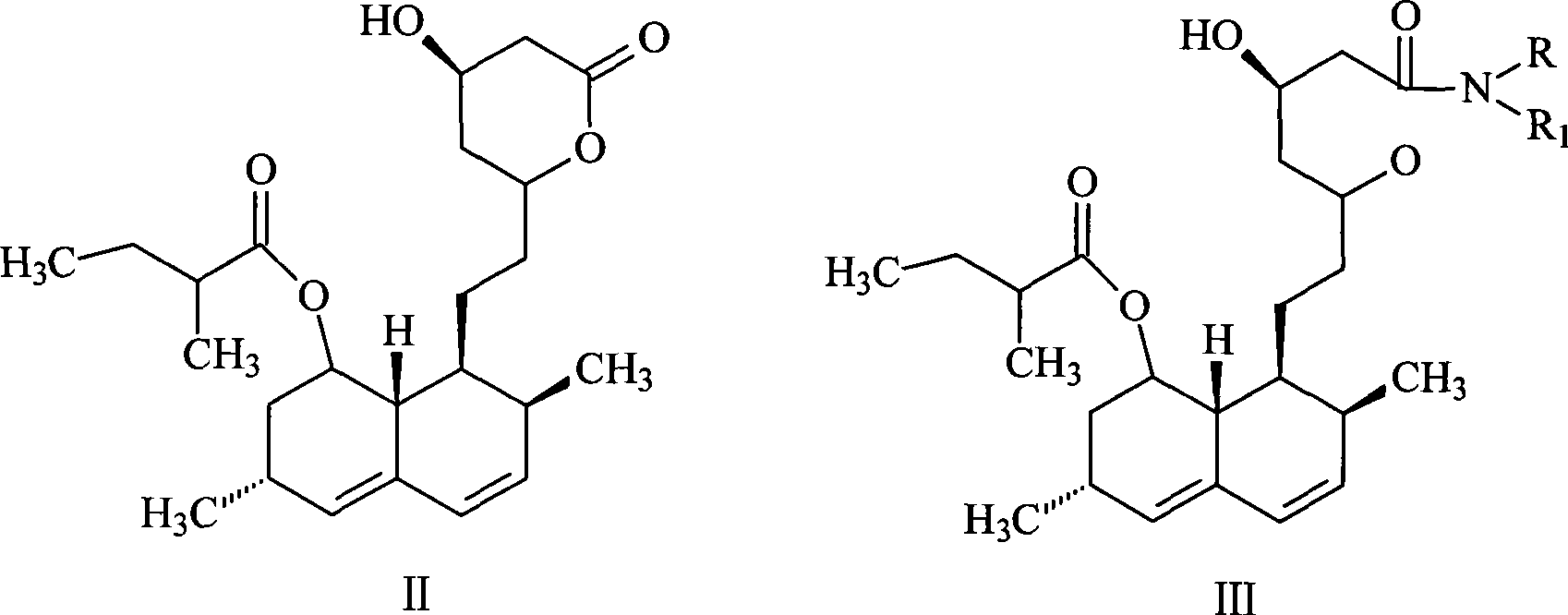
[0032] Synthesis of lovastatin amide (III): ring-opening lovastatin (II) with primary or secondary amines to form lovastatin amide, the reaction temperature is 50-90°C, the reaction time is 2-8 hours, and then the Pressure distillation to remove excess amine to obtain viscous lovastatin amide; or after extraction with an aprotic organic solvent, pickling to remove excess amine, distilling off the solvent to obtain viscous lovastatin amide.
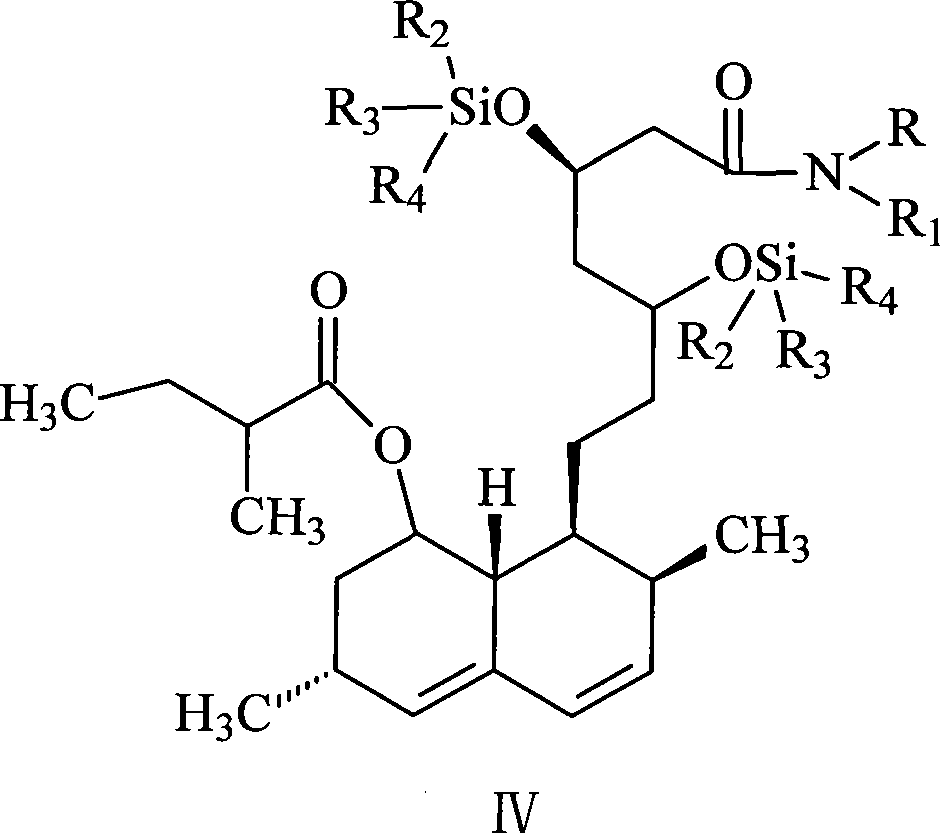
[0033] The synthesis of lovastatin amide bis(trimethylsilyl) ether (VIII): lovastatin amide is dissolved in the composite solvent of tetrahydrofuran and cyclohexane, wherein the volume ratio of tetrahydrofuran and cyclohexane in the composite solvent is (1.0~ 4.2):1, the preferred volume ratio is (2.5~3.5):1. Then, add 1-8 moles of trialkylchlorosilane and 1-6 moles of imidazole, and react at 40-90° C. for 2-10 hours. A better process formula is 4-8 moles of trialky...
Embodiment 1
[0039] (1), N-butyl-7-[1,2,6,7,8α(R)-hexahydro-2(S), 6(R)-dimethyl-8-[[2-(S )-methylbutyryl]oxyl]-1-(S)-naphthyl]-3(R),5(R)-dihydroxyheptanoic acid amide (lovastatin n-butyramide, III) synthesis:
[0040] At room temperature, 24 g (59.6 mmol) of lovastatin and 50 mL of n-butylamine were added into a dry reactor filled with nitrogen, heated to 80° C. and refluxed for 3 to 4 hours. Then cool to room temperature, place in a water bath at 60°C, distill under reduced pressure to remove n-butylamine, or extract with an aprotic organic solvent, then pickle to remove excess amine to obtain viscous lovastatin n-butyramide.
[0041] (2), N-butyl-7-[1,2,6,7,8α(R)-hexahydro-2(S), 6(R)-dimethyl-8-[[2-(S )-methylbutyryl]oxy]-1-(S)-naphthyl]-3(R),5(R)-bis(trimethylsilyloxy)heptanoic acid amide (lovastatin n-butyramide Synthesis of bis(trimethylsilyl) ether, VIII):
[0042] Add 100mL tetrahydrofuran and 50mL cyclohexane into the above viscous substance, after completely dissolving, add 225...
Embodiment 2
[0052] Except step (2), other is with embodiment 1, and the synthesis technique of step (2) is as follows:
[0053] Add 107mL tetrahydrofuran and 43mL cyclohexane to the above-mentioned viscous substance, after completely dissolving, add 238g (3.5 moles) of imidazole and 432g (4.0 moles) of trimethylchlorosilane, heat to 60°C for 3 hours, cool to At room temperature, put it in the refrigerator to refrigerate until the insoluble matter is completely precipitated, then filter, and the filtrate is directly used in the next step of the synthesis reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com