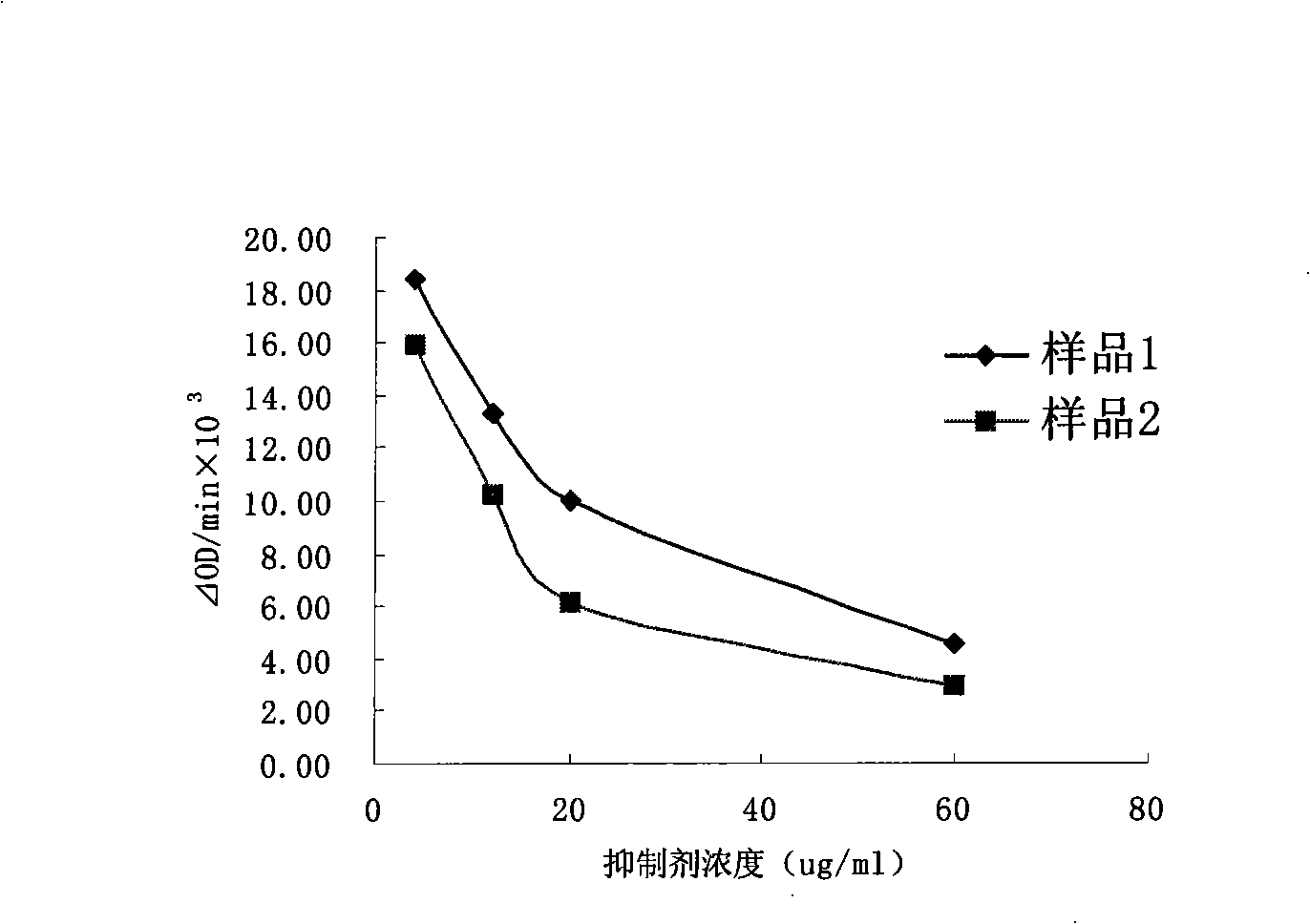
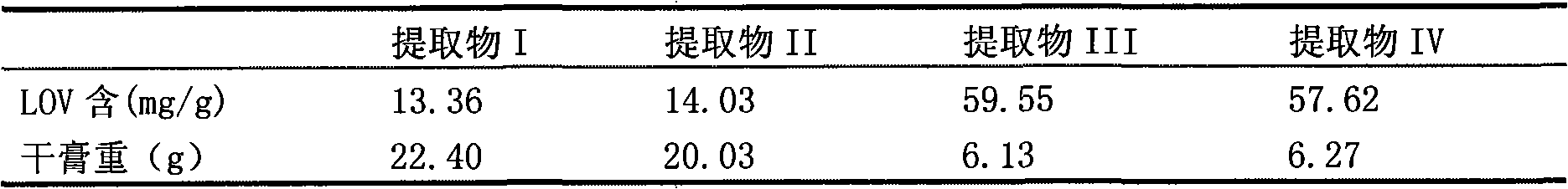
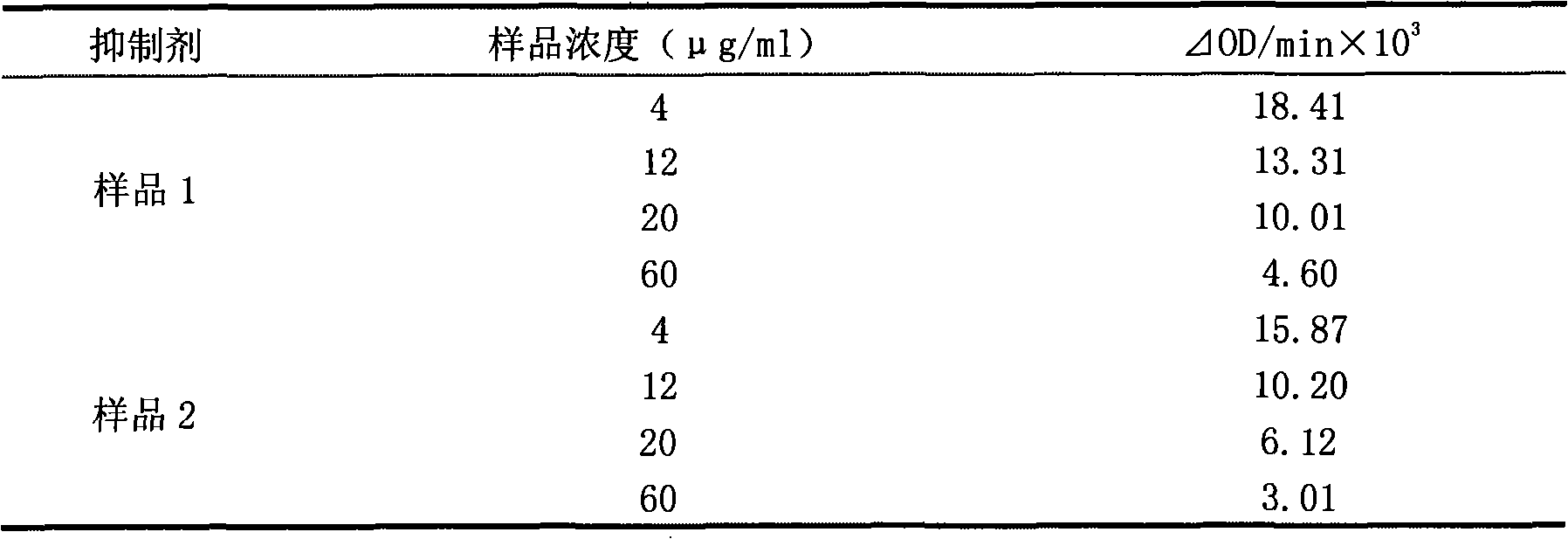
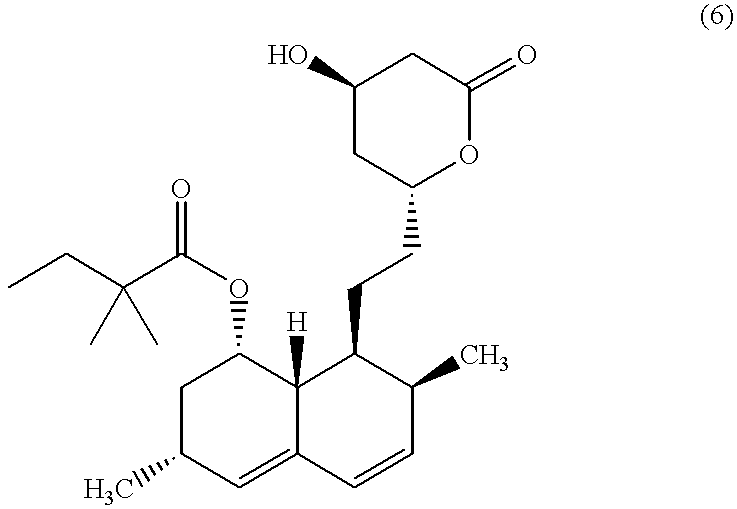
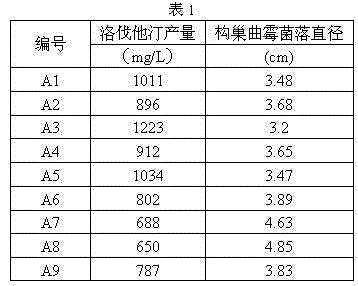
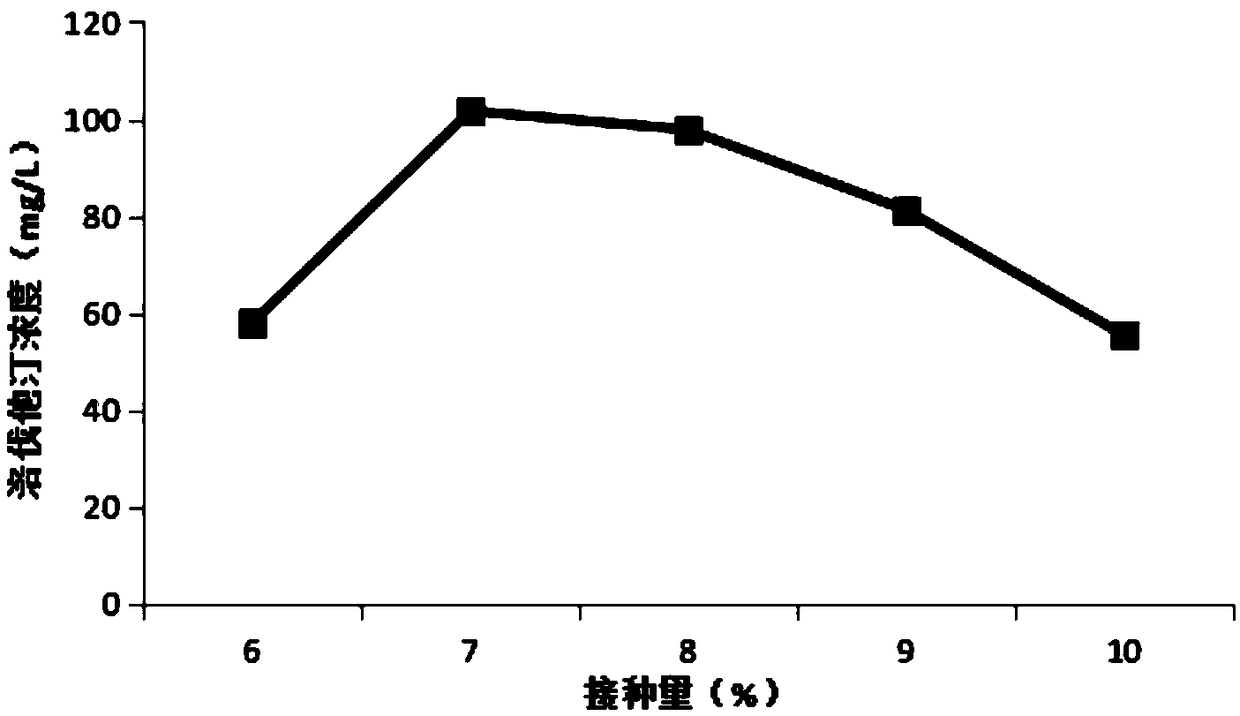
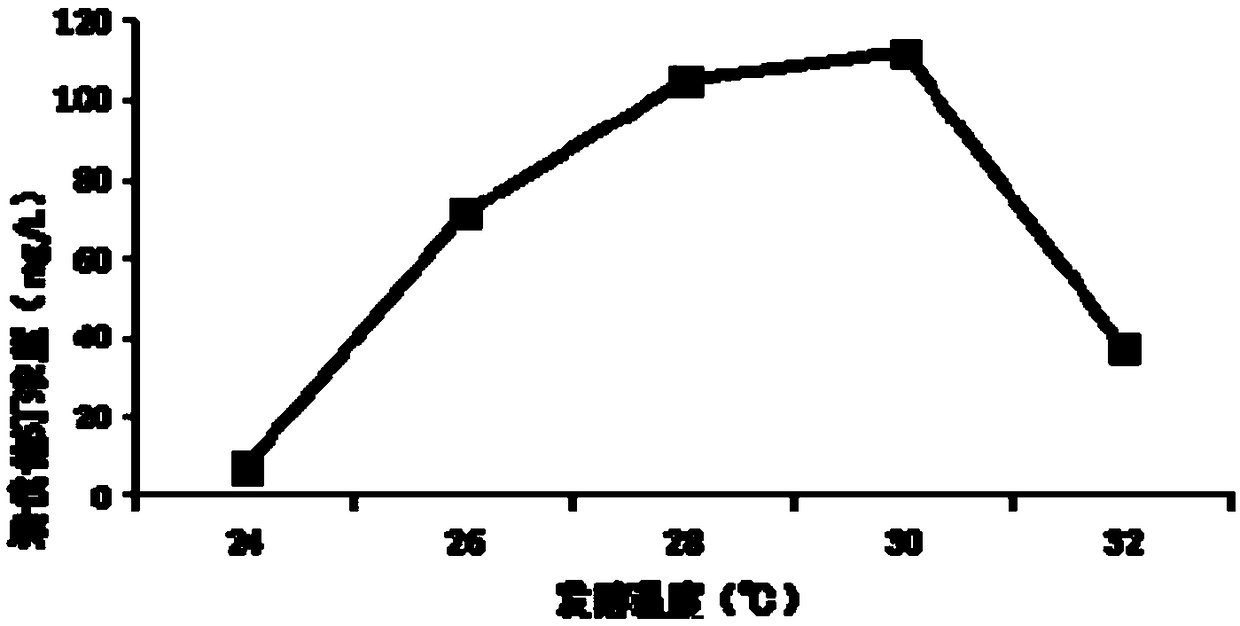
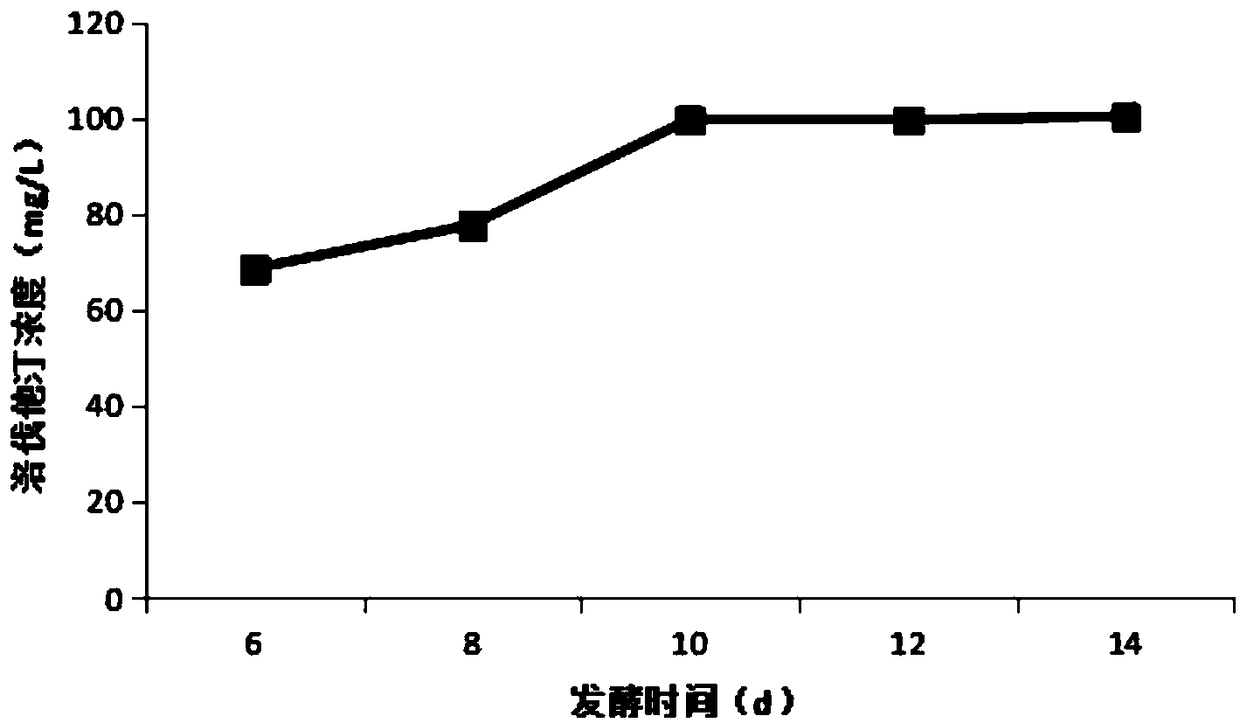
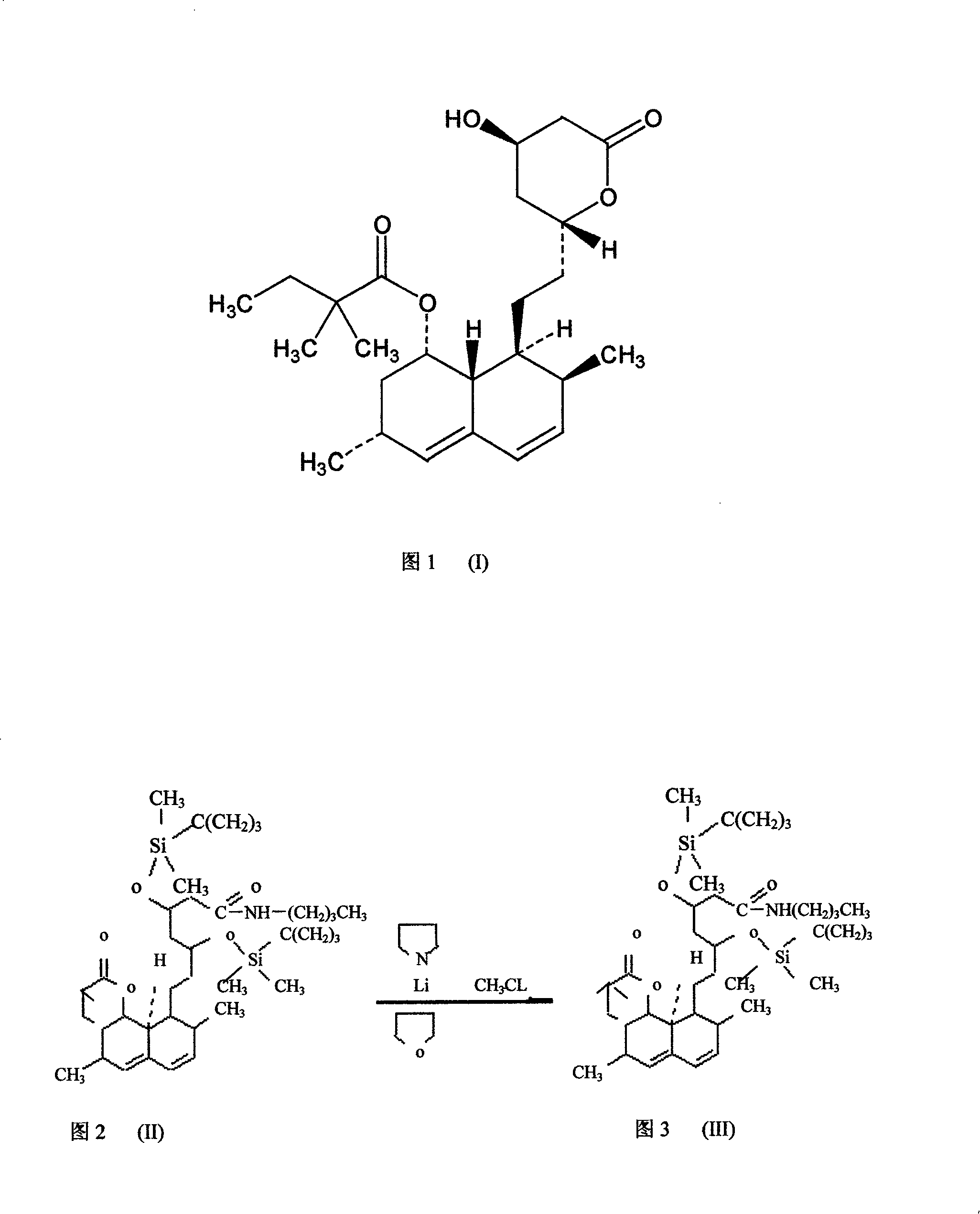
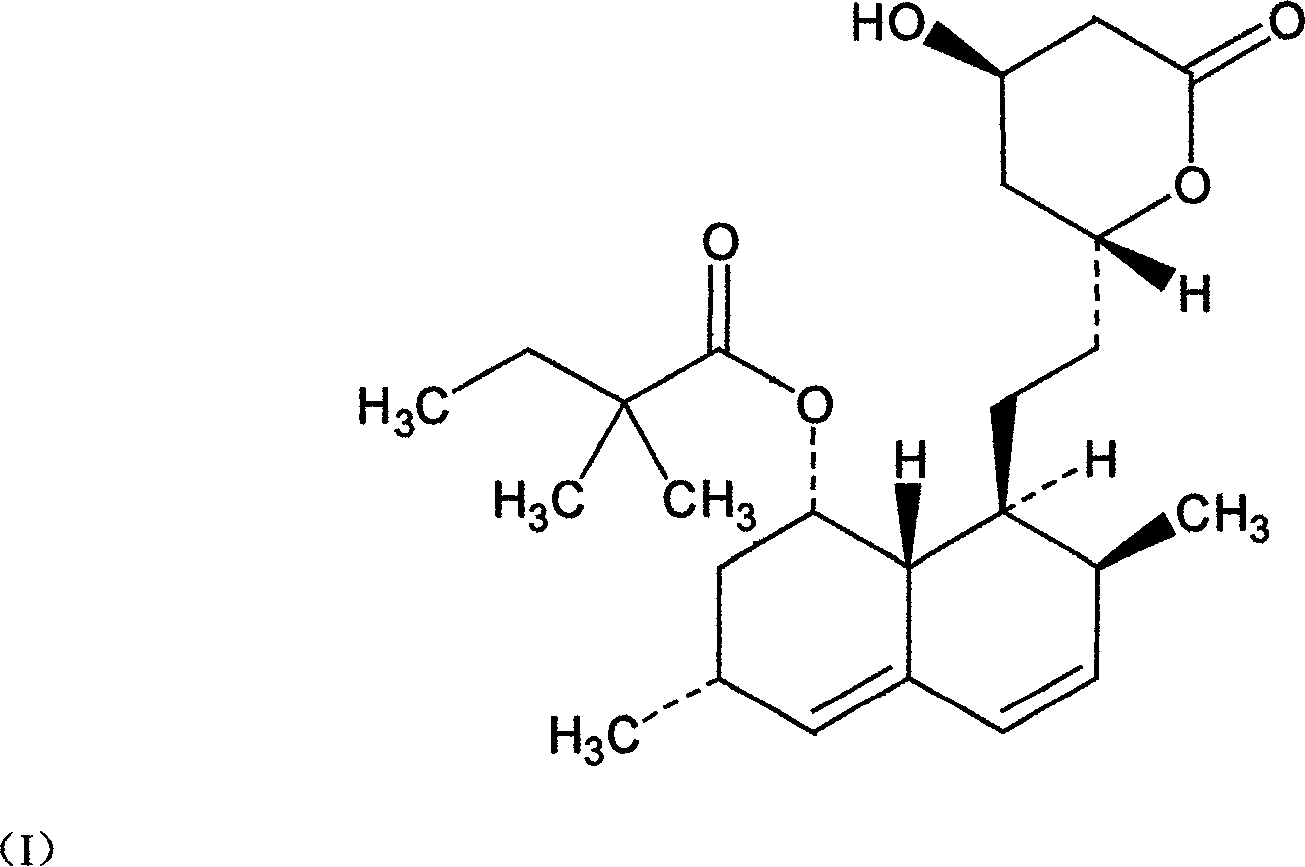
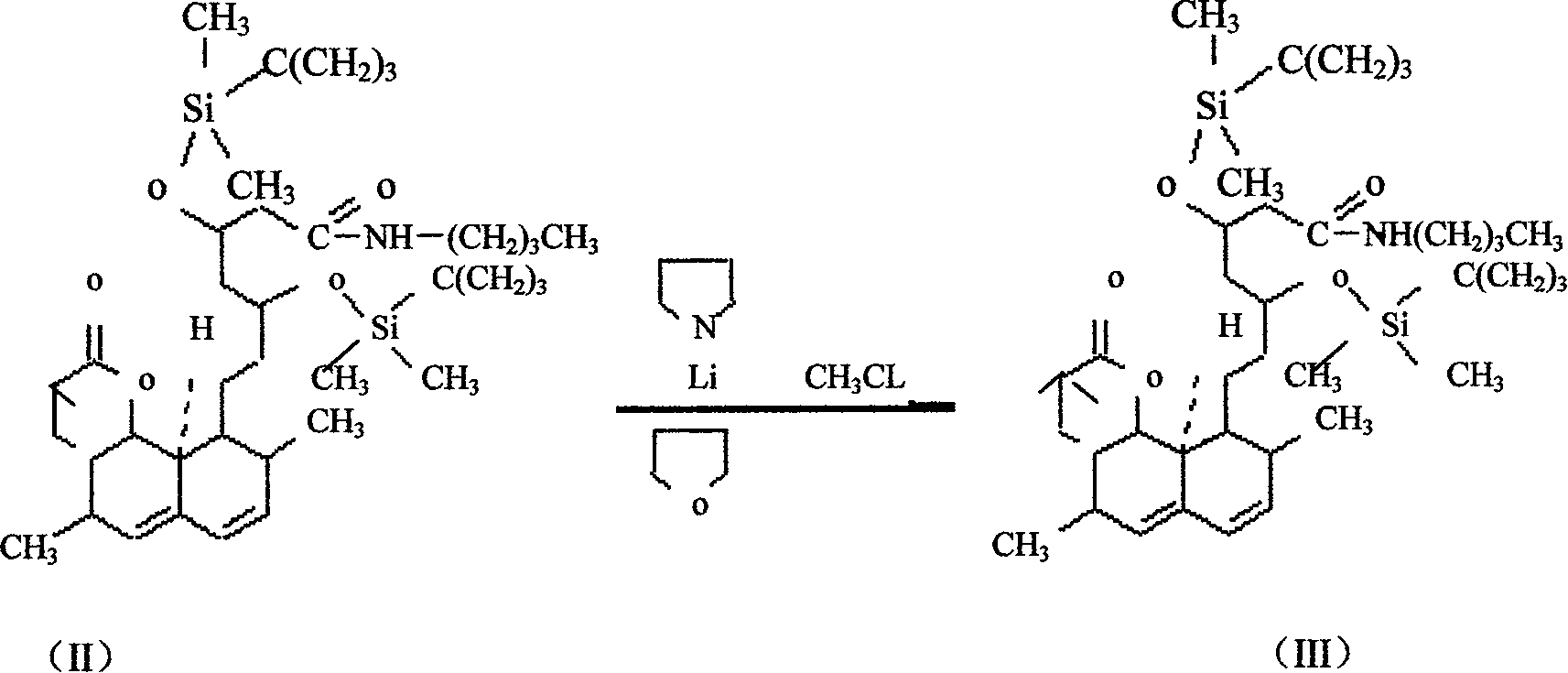
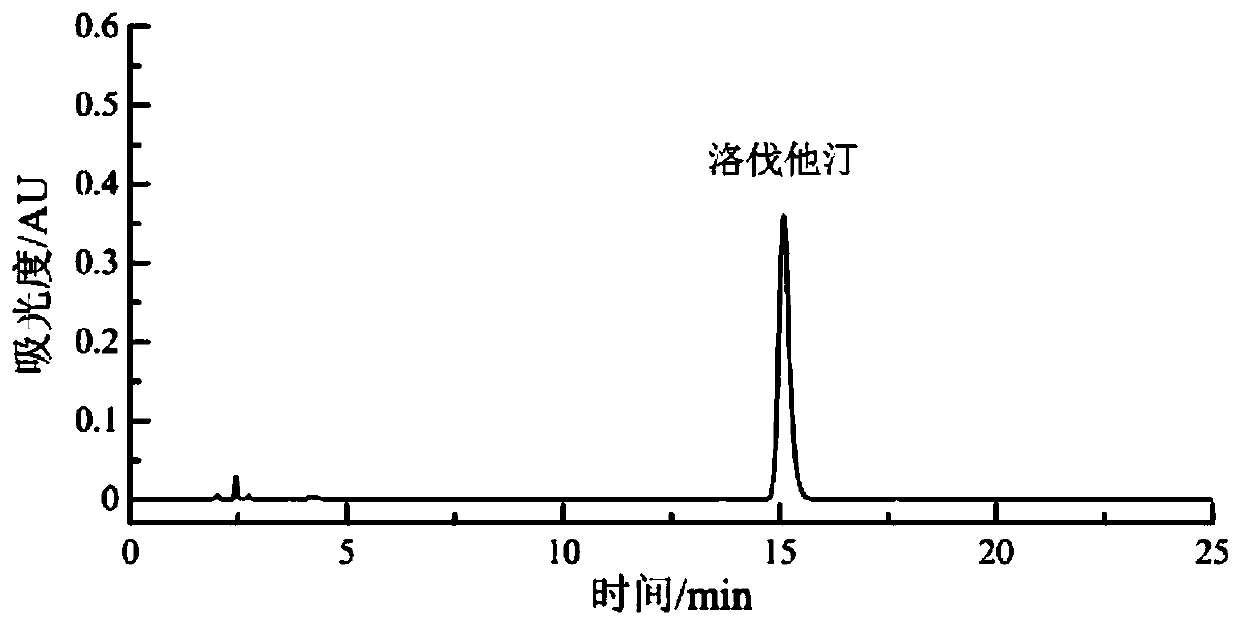
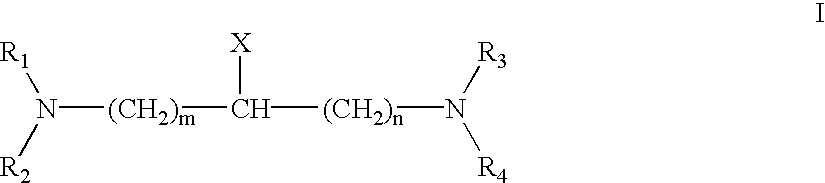
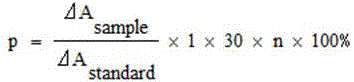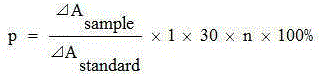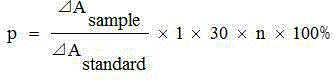Patents
Literature
142 results about "Lovastatine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Rice fermented with red yeast extract, preparation method and quality detection method thereof
ActiveCN101313919AMetabolism disorderMaterial analysis by observing effect on chemical indicatorLovastatinAdditive ingredient
The invention discloses a red yeast rice extract and a preparation method and a quality test method thereof. The main active ingredient of the extract is lovastatin, the content of which is more than 3 percent. The extract is prepared by subjecting a medical material of red yeast rice to extraction with an organic solvent, water decoction after the volatilization of the solvent, precipitation collection by siphonage and configuration, mixing with excipients and drying. The preparation method improves the content of lovastatin. The invention also provides a quality control method for identifying the ingredients and measuring content.
Owner:BEIJING WBL PEKING UNIV BIOTECH
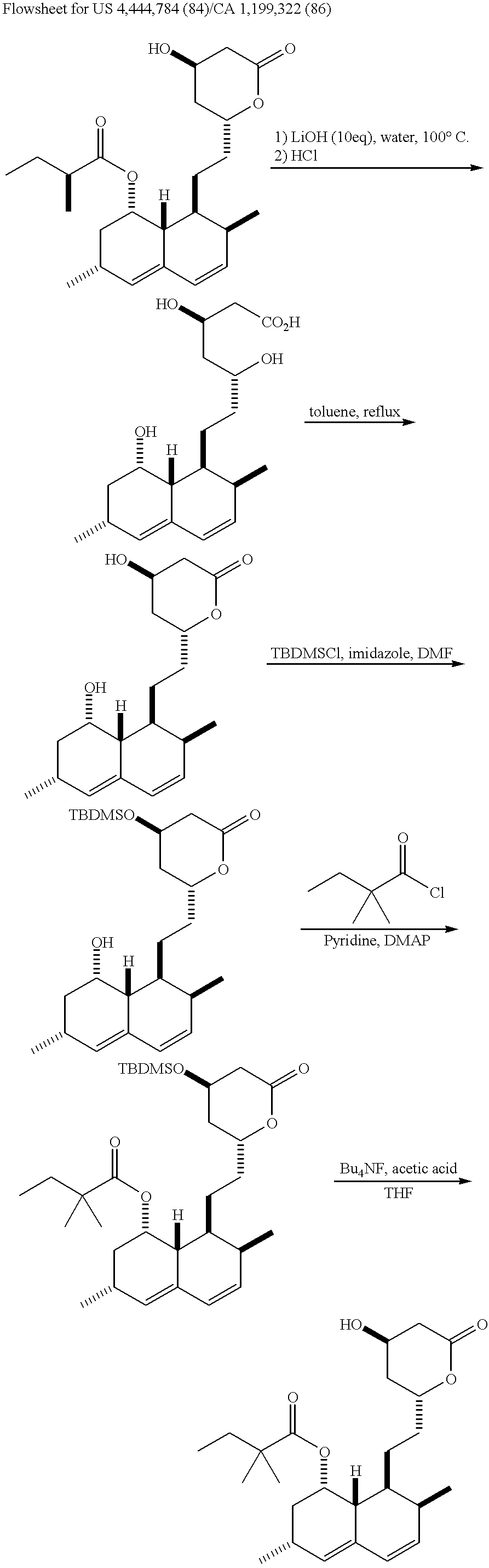
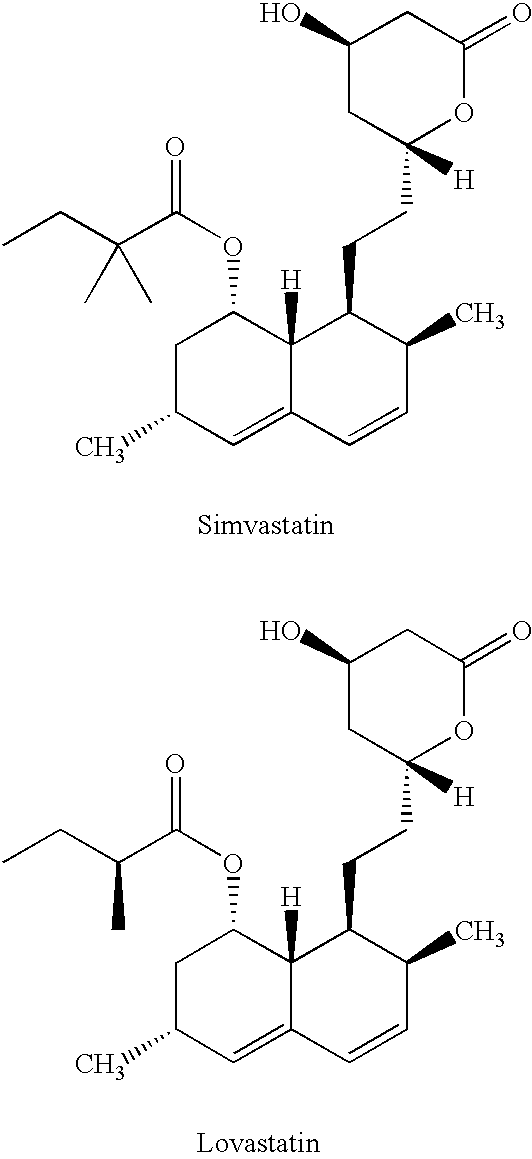
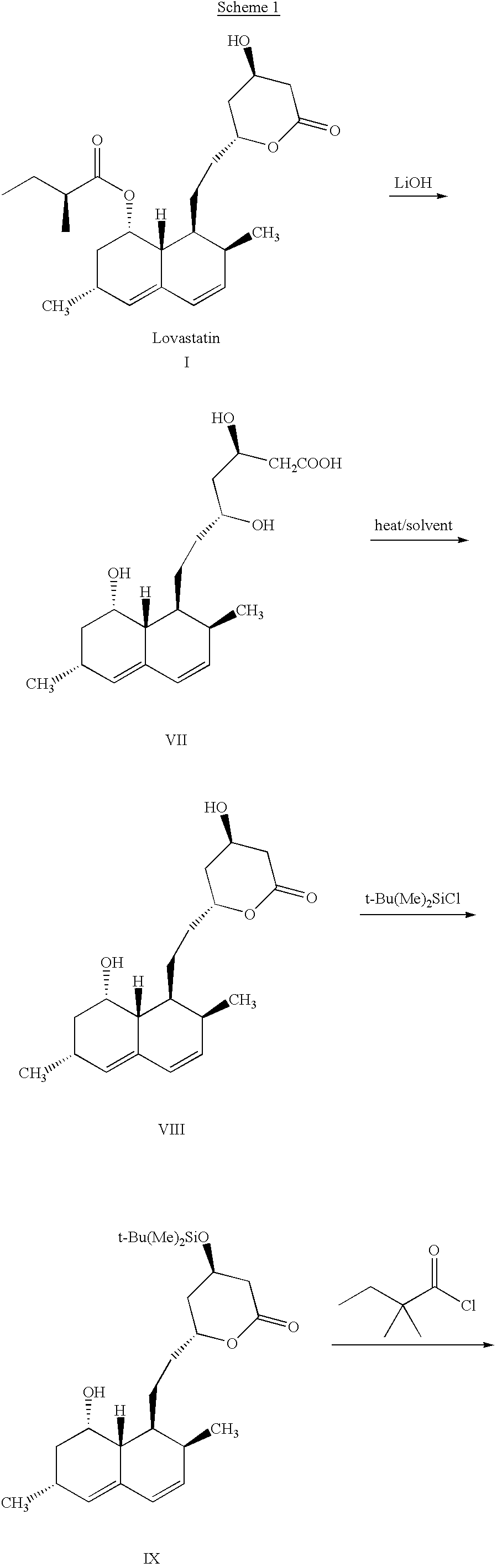
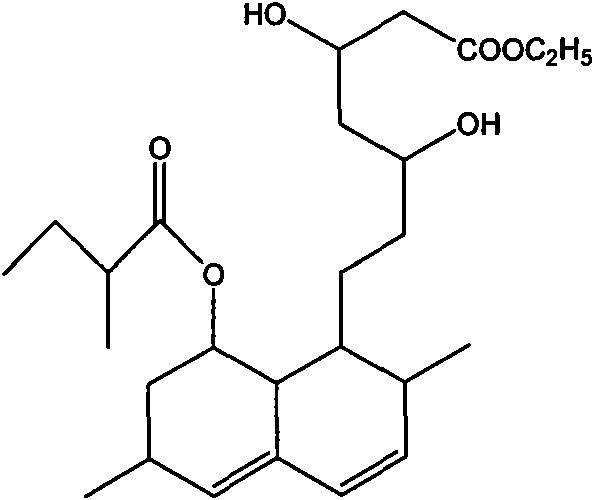
Process for producing simvastatin
InactiveUS6331641B1Improve efficiencyMild conditionsOrganic chemistryBulk chemical productionHMG-CoA reductaseAlcohol
This invention provides an easy and efficient process for producing a simvastatin of great use as an HMG-CoA reductase inhibitor, which comprises deacylation of lovastatin with an inorganic base and a secondary or tertiary alcohol and subjecting the resulting diol lactone to selective protection with a ketal or acetal protective group, acylation and deprotection-lactonization to give simvastatin.
Owner:KANEKA CORP
Manufacturing process, such as three dimensional printing, including binding of water-soluble material followed by softening and flowing and forming films of organic-solvent-soluble material
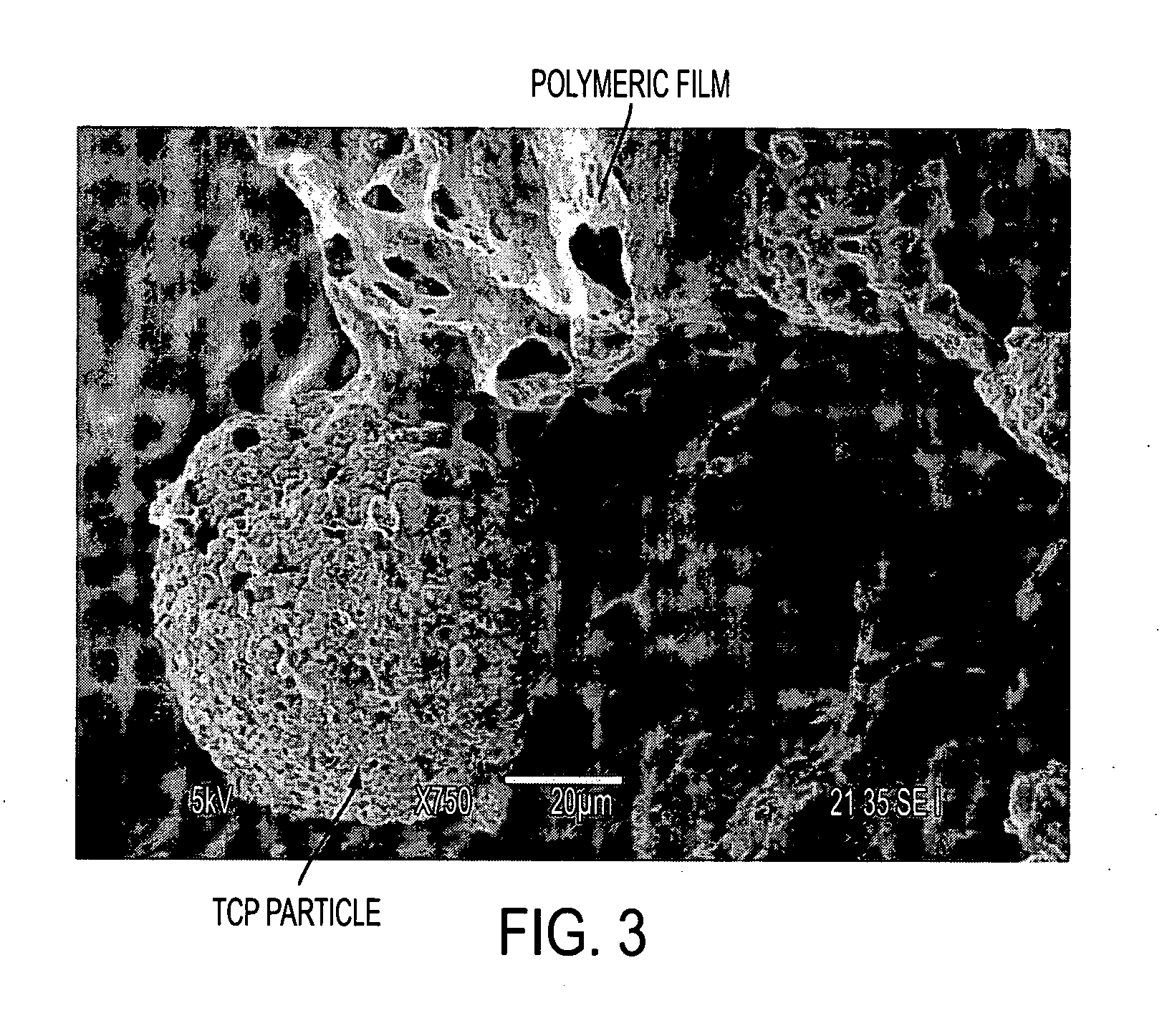
InactiveUS20070009606A1Effectively osteoinductiveHigh porosityAdditive manufacturing apparatusPhotosensitive materialsManufacturing technologyAdditive ingredient
The invention includes biostructures which may be characterized as having substantially all of the organic-solvent-soluble material in the form of a network of irregularly shaped perforated films. The biostructure may further include particles of a substantially-insoluble material, which may be a member of the calcium phosphate family. The biostructure may be osteoconductive. The biostructure may further contain an Active Pharmaceutical Ingredient or other bioactive substance. The API may be a substance which stimulates the production of bone morphogenetic protein, such as Lovastatin or related substances, thereby making the biostructure effectively osteoinductive. One or more of the polymers may have a resorption rate in the human body such as to control the release of the API. Methods of manufacture are also disclosed.
Owner:MASSACHUSETTS INST OF TECH +1
Process for producing simvastatin
InactiveUS6307066B1Silicon organic compoundsGroup 3/13 element organic compoundsLovastatinBoric acid
A process for manufacturing Simvastatin is provided comprising reacting lovastatin with an organic boronic acid to produce a derivative of lovastatin (lovastatin phenylboronate) methylating the 2-methylbutyryloxy group on the lovastatin derivative to form a 2,2-dimethylbutyryloxy group on the lovastatin derivative and thereafter removing the boronate group to produce simvastatin.
Owner:APOTEX PHARMACHEN INC
Monascus fagopyrum-tataricum tea and preparation method thereof
The invention provides monascus fagopyrum-tataricum tea prepared by taking monascus and fagopyrum tataricum as main raw materials. The characteristics of the monascus fagopyrum-tataricum tea comprise that the monascus fagopyrum-tataricum tea is ruddy in color, keeps delicate fragrance of fagopyrum tataricum, is good in mouthfeel, also is relatively high in both lovastatin content and total flavonoid content, and has the substantial effects of reducing blood pressure, reducing blood fat, reducing cholesterol, improving immunity and the like under the synergic effect of monascus and fagopyrum tataricum.
Owner:耿福能
High-yield production method of lovastatin
ActiveCN104561166AIncrease production capacityQuality improvementMicroorganism based processesFermentationBiotechnologyMicroorganism
Owner:山东中惠生物科技股份有限公司 +1
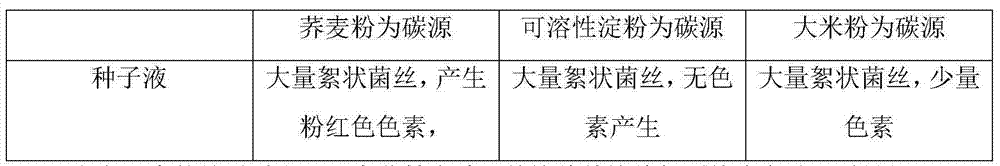
Monascus purpureus strain, and fermentation product thereof and fermentation method thereof
ActiveCN109022293AIncrease productionComply with food and drug safety standardsFungiMicroorganism based processesFood safetyLovastatin
The invention relates to a Monascus purpureus strain, and a fermentation product and a fermentation method thereof. The Monascus purpureus has a strain number of ZX26 and an accession number of CGMCCNO. 15992, and has the advantages of high lovastatin yield, low citrinin synthesis amount, compliance with food safety standards and good genetic stability. The fermentation product comprises myceliaproduced by fermentation and a fermented product lovastatin, and has the advantages of high lovastatin content, low citrinin content, compliance with food safety standards and high nutritional and health-care value. The method comprises a step of inoculating the seed solution of the Monascus purpureus strain into a fermentation medium for oscillating fermentation culture. The fermentation method is simple in operation, low in cost, and convenient for large-scale production. Furthermore, after the fermentation method is optimized, the ability of the Monascus purpureus strain in producing lovastatin can be greatly enhanced.
Owner:BEIJING BEINONG HONGZE BIOTECH CO LTD
Process to manufacture simvastatin and intermediates
InactiveUS6506929B1Reduce usageSimplifies isolationOrganic compound preparationPreparation by transesterificationLovastatinLactone formation
A process is disclosed for the preparation of simvastatin which enables highly regio selective C-methylation of the 2'-position group of lovastatin without requiring protection / deprotection of 13-OH of lovastatin and lactone ring opening / closure.
Owner:APOTEX TECH INC
Method for utilizing monascus to convert yam to produce functional food
InactiveCN106136136AAvoid inhibitionNo pollution in the processFungiMetabolism disorderLovastatinSolid substrate
The invention discloses a method for utilizing monascus to convert yam to produce a functional food and relates to the technical field of bioengineering. According to the preparation method, sequentially through the steps of enlarged culture in a test tube, liquid shake-flask culture and enlarged culture in a seed tank, solid fermental cultivation, drying and smashing, the functional food which regulates blood lipids, blood sugar and blood pressure, resists radiation and inhibits tumor is obtained. According to the method, the yam, rice, wheat, corn and sorghum and the like are taken as solid substrates, monascus is taken as a strain to convert yam saponins through solid fermentation, monascus pigment and monascus polysaccharides are synthesized; in the obtained product, the content of the saponins is 2-20 mg / g of dry substrate, the content of the monascus polysaccharides is 10-100 mg / g of dry substrate, the content of lovastatin is 0.5-10 mg / g of dry substrate, the content of the monascus pigment is 1-20 mg / g of dry substrate, and the method can be used for extracting the saponins, the monascus polysaccharides, lovastatin and the monascus pigment and producing tablets or capsules for treating blood lipids, blood sugar and blood pressure, resisting radiation and inhibiting tumor.
Owner:JIANGSU UNIV
Method for producing lovastatin by fermentation and fermentation medium used by same
InactiveCN102559795AIncrease productionShorten the fermentation cycleMicroorganism based processesFermentationLovastatinGlucose polymers
The invention provides a method for producing lovastatin by fermentation and a fermentation medium used by the same. The fermentation medium takes maltose as a carbon source and does not contain glucose. The method for producing lovastatin by fermentation by using the fermentation medium is characterized in that: Aspergillus terreus for producing lovastatin is cultured by fermentation in the fermentation medium provided by the invention. The method provided by the invention improves the fermentation unit of lovastatin by about 10%, simultaneously shortens the fermentation period, lowers the production cost, and is suitable for industrialized production.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
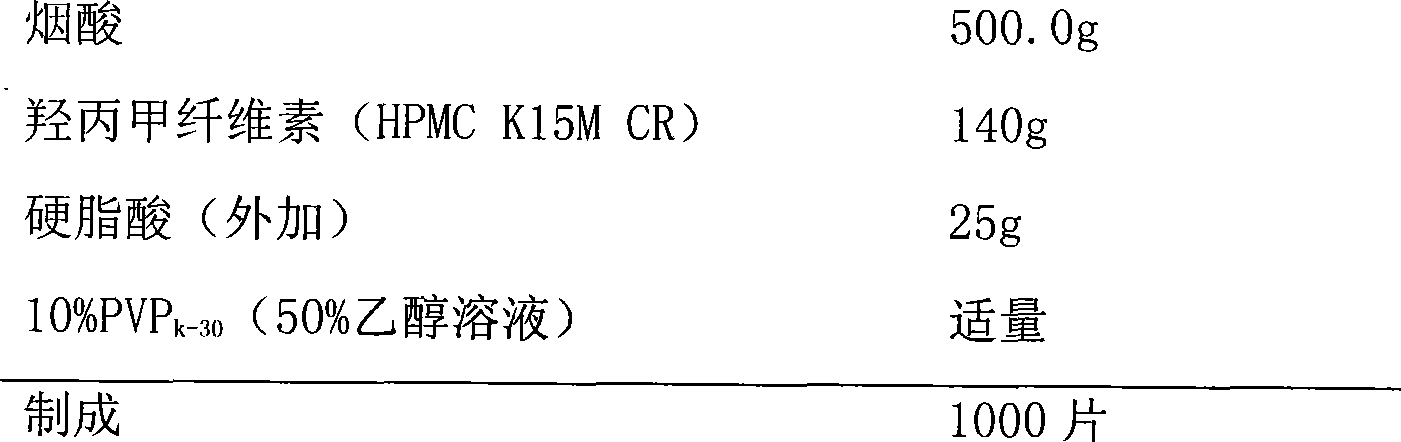
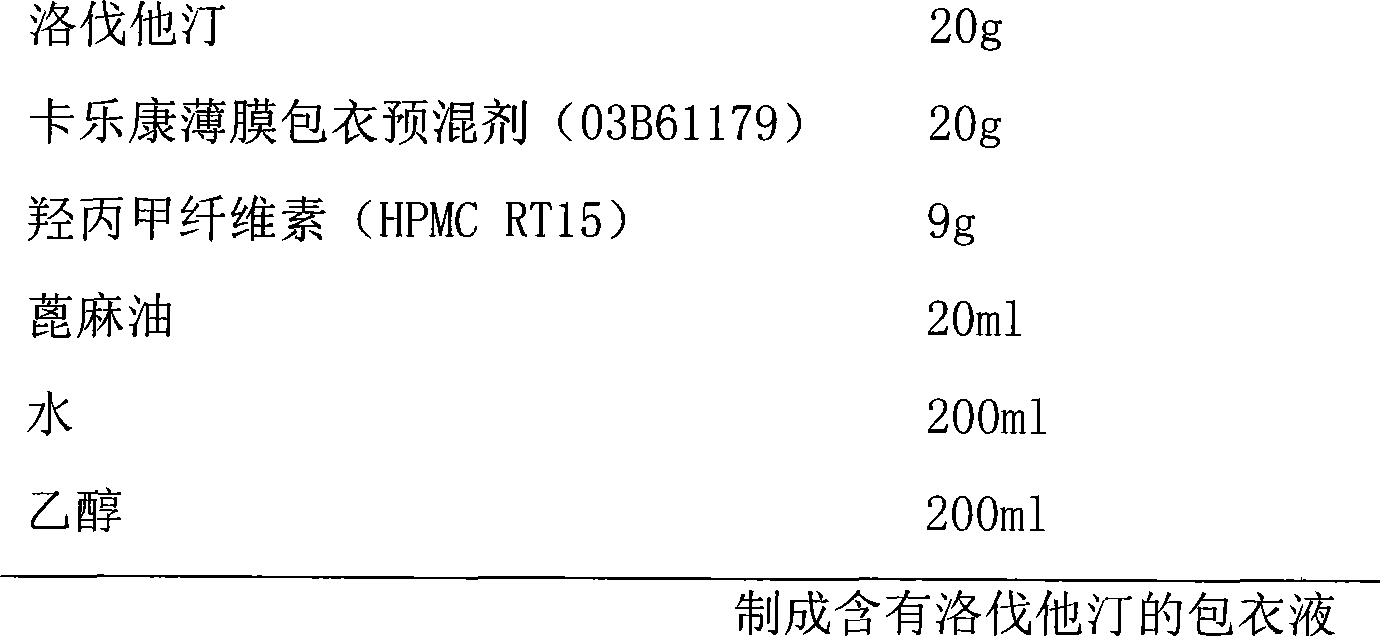
Lovastatin and niacin slow-release tablet and preparation method thereof
InactiveCN101390843AOutstanding lipid-lowering effectSmall fluctuations in blood concentrationMetabolism disorderPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention relates to a lovastatin and niacin sustained-release tablet used for reducing blood lipid and the preparation method thereof; each tablet contains 500mg of niacin and 20 mg of lovastatin. The coating form is adopted for preparation; the niacin is used as the core of the sustained-release tablet; lovastatin is dissolved in coating liquid to be used as quick release part. The lovastatin and niacin sustained-release tablet used for reducing blood lipid and the preparation method have the advantages of simple and practical preparation process, low cost, enabling the medicine release to accord with the national pharmacopoeia and the treatment requirements, well improving the clinical symptoms, reducing side effects and providing a blood-reducing medicine with low price.
Owner:SHAANXI BUCHANG PHARMA
Nanoparticulate statin formulations and novel statin combinations
InactiveUS20110027371A1Increase bioavailabilityIncreased rate of dissolutionPowder deliveryBiocideDrugStatine
Owner:ELAN PHRMA INT LTD
Red yeast rice rich in coenzyme Q10 and preparation method thereof
ActiveCN103815279AEasy to synthesizeWide range of usesCosmetic preparationsToilet preparationsBiotechnologyRed yeast rice
The invention discloses a red yeast rice rich in coenzyme Q10 and a preparation method thereof. The method comprises the following steps: using monascuspilosus and red monascus as fermented strains, using polished round-grained rice and indica rice as main raw materials, using saccharose, red chilli powder and linoleic acid as auxiliary materials, and conducting segmented temperature control culturing to form the red yeast rice rich in coenzyme Q10. The method utilizes double-strain mixed fermentation and combines temperature control culturing to prepare the red yeast rice rich in coenzyme Q10, and the method is simple, and easy to control. The content of coenzyme Q10 of the prepared red yeast rice can achieve 400-550mg / kg, and the prepared red yeast rice does not contain citrinin. Meanwhile, the red chilli powder and the linoleic acid are added in the preparation process so as to promote the synthesis of lovastatin and gama-aminobutyric acid, and the red yeast rice rich in coenzyme Q10 can be used as a raw material to be widely applicable to processing of foods, medicine and cosmetics.
Owner:FUJIAN AGRI & FORESTRY UNIV
Method for preparing lotus seed functional fermented food
The invention provides a method for preparing a lotus seed functional fermented food, relating to the field of microbial fermentation and biological medicine. According to the method, monascus and lactobacillus serving as fermenting strains are subjected to cant activated culture and liquid expansion culture, are inoculated to a lotus seed fermentation culture medium, and are fermented to obtain a functional fermented product containing alkaloids, flavone, lovastatin and monascus color. The fermented product prepared by the method is dried and ground to prepare the lotus seed functional fermented food with multiple effects of regulating blood glucose and blood lipid, improving gastrointestinal functions and improving body immunity.
Owner:FUJIAN AGRI & FORESTRY UNIV
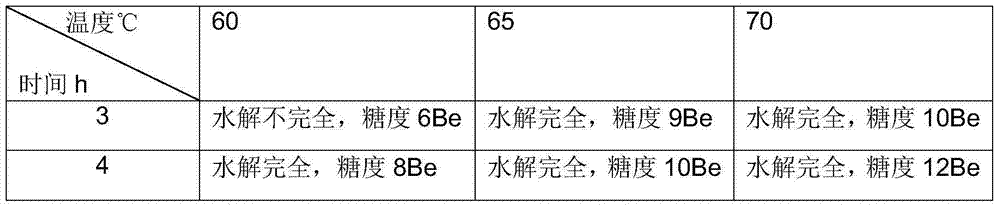
Preparation method and application of monascus transformed tartary buckwheat fermentation liquor
The invention discloses a preparation method and application of monascus transformed tartary buckwheat fermentation liquor, and belongs to the field of biological fermentation and medicine. Monascus is adopted as an original strain, liquid shake-flask culture and liquid seed enlarge cultivation are carried out, and the fermentation liquor containing flavone, lovastatin and monascus is obtained through liquid fermentation. The fermentation liquor contains 20 mg / L to 100 mg / L of flavone, 5 mg / L to 50 mg / L of lovastatin and 4 mg / L to 60 mg / L of monascus. The fermentation liquor produced through the method can be used for producing healthcare food for adjusting blood sugar and blood fat, or the active ingredients such as flavone, lovastatin and monascus of the fermentation liquor are extracted for producing drugs for treating hyperlipidemia and diabetes.
Owner:JIANGSU UNIV
Monascus purpureus transformed corn bran fermentation liquid preparation method and application thereof
The present invention discloses a monascus purpureus transformed corn bran fermentation liquid preparation method and application thereof, belonging to the field of biological fermentation and medicines. The monascus purpureus transformed corn bran fermentation liquid uses monascus purpureus as a starting bacterium strain to conduct a liquid shake-flask culture, a liquid seed expanding culture, and a liquid fermentation to obtain a fermentation liquid containing polysaccharides, lovastatin and monascus pigment. The fermentation liquid contains polysaccharides 100-500 mg / L, lovastatin 5-50 mg / L, and monascus pigment 4-60 mg / L. Therefore the produced fermentation liquid can be used in the production of health-care food which can regulate blood sugar and blood lipids. Besides, the active ingredients of the polysaccharides, lovastatin and monascus pigment in the health-care food can be extracted to produce medicines which can cure patients with high cholesterol and diabetes.
Owner:JIANGSU UNIV
Method for synthesizing statins compounds
InactiveCN101190907ASimple processReduce manufacturing costOrganic chemistryDisiloxaneMethylating Agent
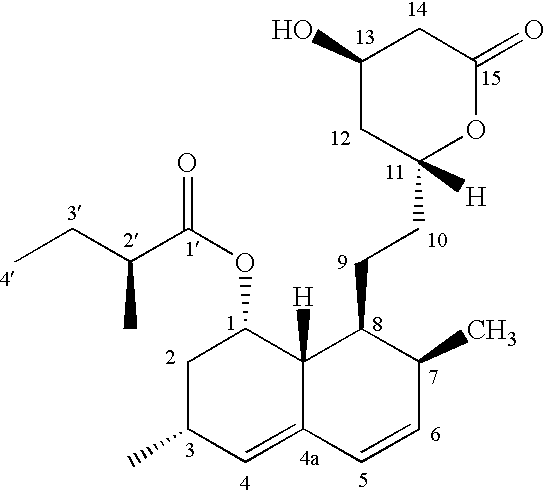
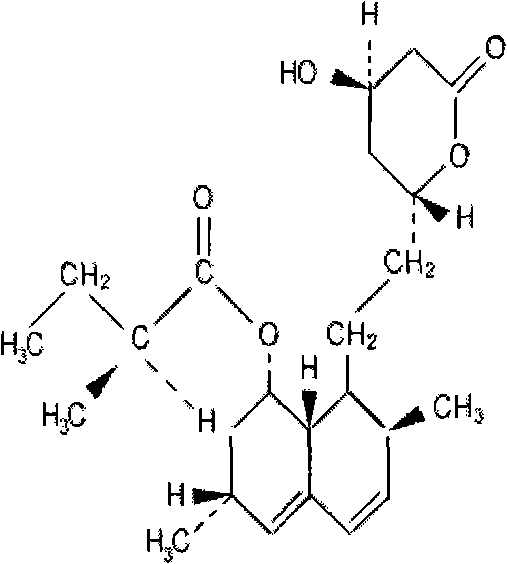
The invention relates to a novel synthesis method of statin compounds, in particular to a preparation method of methylation reaction of Simvastatin (2,2-dimethylbutyric acid-8-((4R, 6R)-6-(2-((1s, 2s, 6R, 8S, 8aR)-1,2,6,7,8,8-a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl)ethyl))tetrahydrochysene-4-hydroxy-2H-pyran-2-ketone ester) and analogues thereof. The method consists of the reaction of Lovastatin amido disiloxane and methylating agent.
Owner:马群力 +1
Preparation method and application of functional monascus-fermented corn bran food
The invention relates to a preparation method and application of a functional monascus-fermented corn bran food, and belongs to the fields of biological fermentation and medicines. According to the preparation method and application of the functional monascus-fermented corn bran food, disclosed by the invention, monascus is used as an original strain for culturing liquid by shaking a flask, liquid seeds are subjected to propagation, and solid fermentation is performed on corn bran, so that functional foods containing lovastatin and monascus pigments are obtained. The food contains 10-50mg / g of polysaccharide, 0.5-5mg / g of lovastatin and 0.4-6mg / g of monascus pigments. The functional food prepared by the method disclosed by the invention can be used for producing health care foods capable of regulating blood pressure and blood fat, or extracting active components, namely the polysaccharide, the lovastatin and the monascus pigments, so as to be used for producing medicines for treating hyperlipaemia and diabetes.
Owner:JIANGSU UNIV
Red yeast rice extract oil and the preparation and application of the soft capsule thereof
ActiveCN101584716AEfficient removalIncrease contentMetabolism disorderFungi medical ingredientsVegetable oilLovastatin
Disclosed is a red yeast rice extract oil and the preparation and application of the soft capsule thereof. The preparation method comprises: adding solvent into the red yeast rice medicinal material, heating for backflow for 2-5 hours; filtering, adding the filter residue into the solvent, heating for backflow for 1-4 hours; merging the filtrates; condensing at 50-90 degrees centigrade into extract with relative density of 1.1-1.4 at 25 degrees centigrade; adding ethanol by amount of 1-4 times (mass) of the red yeast rice medicinal material into the extract; dissolving, filtering, recovering ethanol to obtain stiff paste; adding ethanol by amount of 0.5-1 times (mass) of the red yeast rice medicinal material into the stiff paste; distilling to dryness at 60-95 degrees centigrade, insulating thermal for 1-5 hours; adding 0.5-4 times of ethanol into the extract; adding 0.5-5 times of vegetable oil, stirring uniformly at 50-70 degrees centigrade; recovering the ethanol to obtain the red yeast rice extract oil. The content of the active ingredient: lovastatin in the soft capsule prepared by the red yeast rice extract oil is 0.2-1.2wt%; the soft capsule is applied for preparing medicament for treating hyperlipemia; The invention can remove the large amount of unavailability components in the red yeast rice, and increases the content of lovastatin compounds in the extract of per weight unit effectively.
Owner:云南永安制药有限公司
Purple monascus, method and application thereof for producing lovastatin through co-fermentation
The invention discloses purple monascus, a method and an application thereof for producing lovastatin through co-fermentation, and belongs to the fields of fermentation engineering and biotechnology.The purple monascus is preserved in China Center For Type Culture Collection on December 20, 2018, with the preservation number of CCTCC No. M2018910, and the preservation address of Wuhan University,Wuhan, China. The yield of lovastatin in the monascus starter prepared by the monascus solid-state fermentation can reach 6-12mg / g, the color value can reach 1500-2500u / g, both being at a high levelwithout citrinin detection.
Owner:JIANGNAN UNIV
Composition for prevention and treatment of cardio-cerebrolvascular disease
InactiveCN101380398APrevent obesityPrevent hyperlipidemiaMetabolism disorderFood preparationMedicineLovastatin
The invention relates to a composite which prevents and treats cardiovascular and cerebrovascular diseases. The composite consists of the materials according to the parts by weight: 10 to 200 parts of Konjak refined powder, 1 to 100 parts of hawthorn P. E., 1 to 100 parts of fructus lycii P.E, 1 to 100 parts of mulberry seed P. E. and 0.01 to 5 parts of red rice (counted by lovastatin). The composite can play a positive part in preventing and treating the cardiovascular and cerebrovascular diseases, intervenes in a plurality of forming links of cardiovascular and cerebrovascular diseases and can be used for preparing medicines, health food or food, and the like, which can prevent and treat cardiovascular and cerebrovascular diseases.
Owner:毛晓敏
Method for preparing open-loop lovastatin
InactiveCN101659923AGood choiceHigh chemoselectivityFungiMicroorganism based processesMicroorganismLovastatin
The invention discloses a method for preparing open-loop lovastatin by using monascus purpureus, which is a biological conversion method for utilizing monascus to produce enzyme and hydrolyze closed-loop lovastatin into the open-loop lovastatin. The method comprises the following steps: selecting and culturing a monascus strain CGMCC NO.0272; putting the cultured monascus strain CGMCC NO.0272 intoa fermentation medium for fermentation; preparing crude enzyme solution by a salting out method; performing substrate preparation; performing enzymatic conversion reaction; and performing other steps. The method for preparing the open-loop lovastatin by using the monascus purpureus adopts microbial conversion means, and compared with chemical means, the microbial conversion means has the advantages of moderate condition, simple equipment, less public hazard, quick reaction rate and relatively environmental protection.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Aspergillus clavatus-32 strain capable of highly yielding Lovastatin and application of Aspergillus clavatus-32 strain
ActiveCN105219653AFast growthHigh sporulationFungiMicroorganism based processesBiotechnologyMicroorganism
The invention belongs to the field of biotechnology microorganisms, and particularly relates to an Aspergillus clavatus-32 strain producing Lovastatin through liquid fermentation, application and a fermentation producing method. The Aspergillus clavatus-32 strain is high in Lovastatin yield and growing speed, large in sporulation quantity and simple to culture. Under the conditions that initial pH of a fermentation culture medium is 5.2 and culture temperature is 28 DEG C, after the liquid fermentation culture medium and fermentation conditions are optimized and the Ac-32 strain is fermented in a 5L fermentation tank for 110-140h, yield of Lovastatin can reach about 200-250mg / L.
Owner:ZHEJIANG NORMAL UNIVERSITY
Preparation method of nutrient and functional fermented soybean meal fermented by purebred strains
PendingCN110393266AReduce pollutionRealize energy savingNatural extract food ingredientsFood ingredient functionsLovastatinAglycone
The invention belongs to the technical field of fermentation, and particularly relates to a preparation method of nutrient and functional fermented soybean meal fermented by purebred strains. The preparation method of the nutrient and functional fermented soybean meal fermented by the purebred strains comprises the steps that raw material pretreatment, cooking, drying and fermentation are carriedout, wherein at the beginning of fermentation, the purebred monscus.sp.9901 is accessed. In the fermentation process, the fermented soybean has higher protease activity and isoflavone aglycone conversion rate in the fermentation process, and main physiological active substances produced by the Monascus is lovastatin. The protease activity is 115.47 U.g<-1>; and the isoflavone aglycone content is 77.10%; and the ring-opening lovastatin content and lactone-type lovastatin content are separately 579.12 [mu]g / g and 116.5887 [mu]g / g.
Owner:SHENYANG PHARMA UNIVERSITY
Method for preparing simvastatin intermediate - simva-acylamide second silicon ether
ActiveCN101747357ALow costSimple production processSilicon organic compoundsCardiovascular disorderSide chainLovastatin
The invention relates to a preparation method of the intermediate of medicine simvastatin. The method comprises the following steps: 1) lovastatin and amine react to prepare lova-acylamide; 2) hydroxyl in lova-acylamide molecules is protected; and 3) sulfonic acid single-ester and sulfuric acid ester compounds are used to methylate the pha-carbon of lova-acylamide second silicon ether side chain 2 - methyl butyric ester to obtain the simva-acylamide second silicon ether. The simva-acylamide second silicon ether can be used for preparing simvastatin. The method greatly reduces the cost for preparing the key intermediate of the simvastatin - simva-acylamide second silicon ether, simplifies the production technology and improves product purity.
Owner:NEW FOUNDER HLDG DEV LLC +2
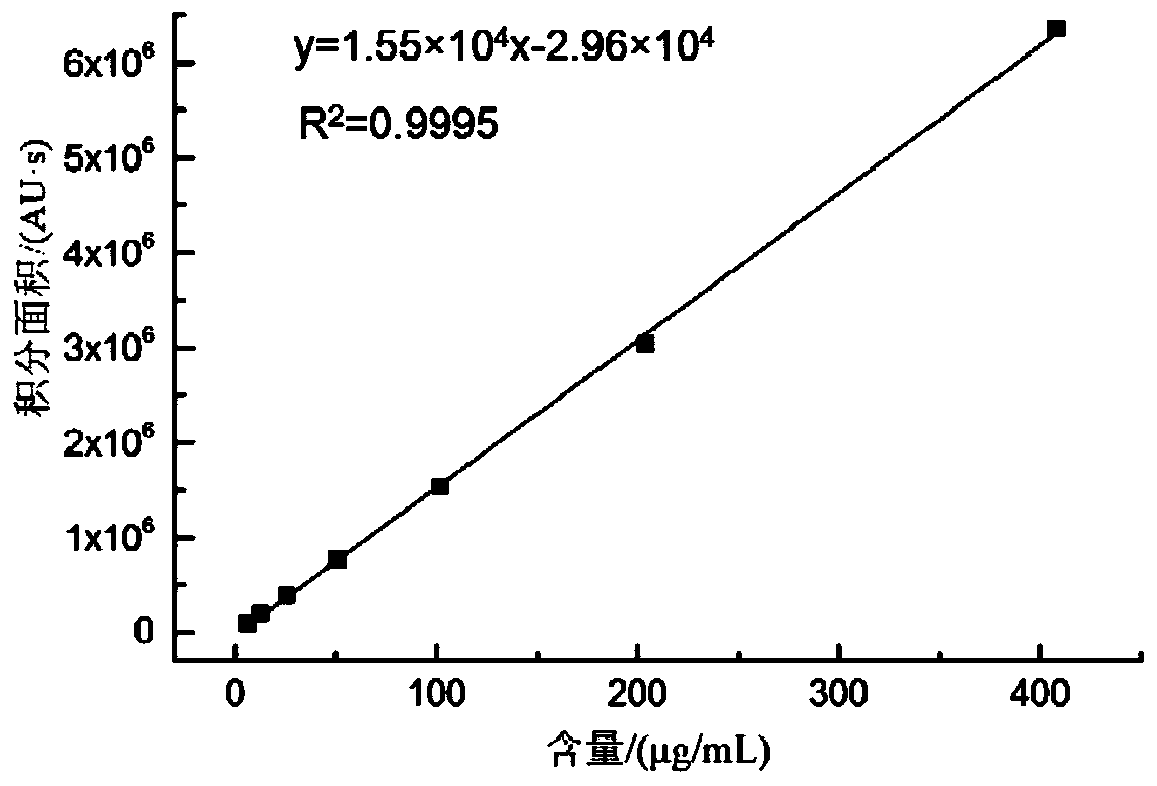
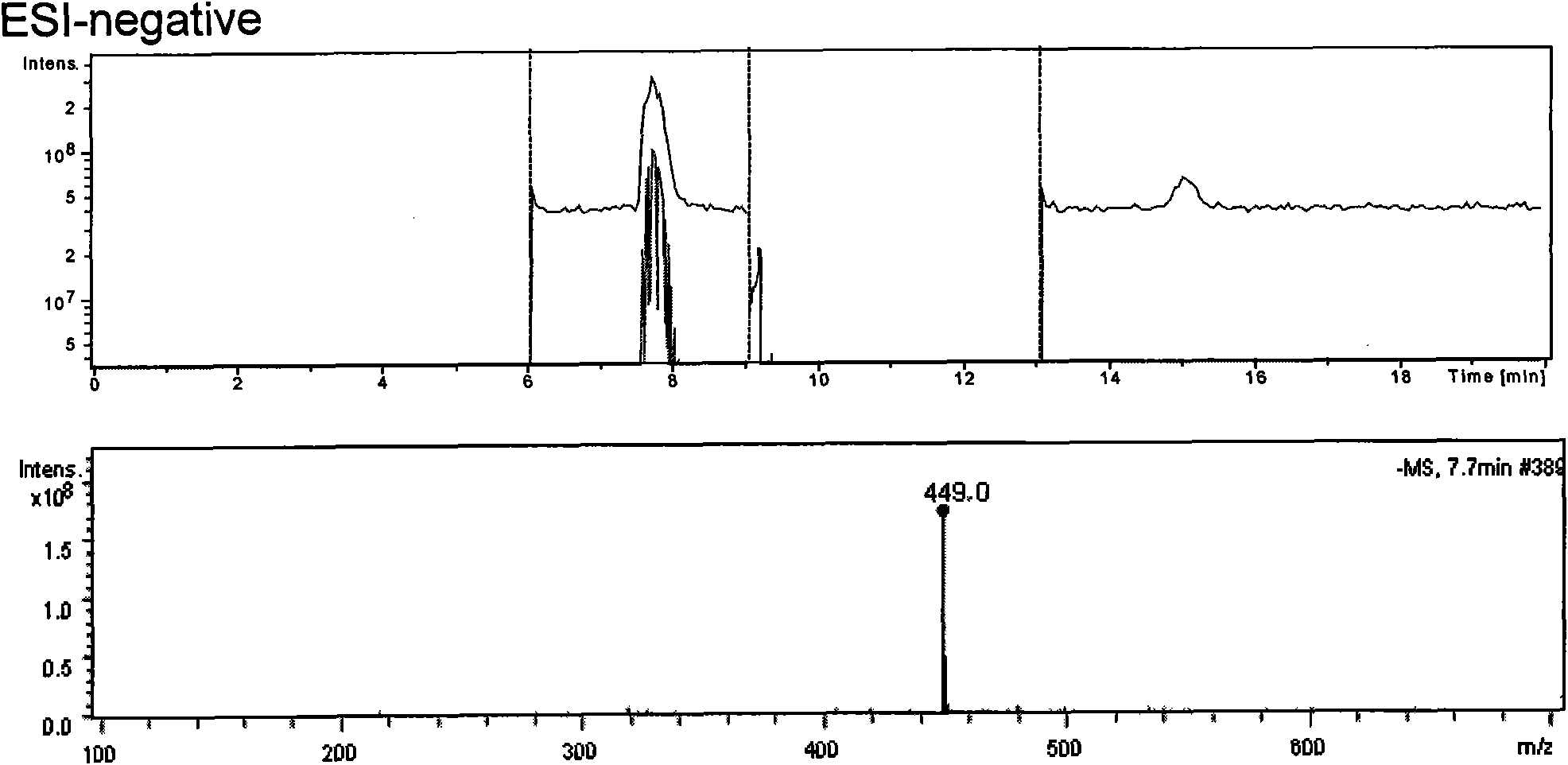
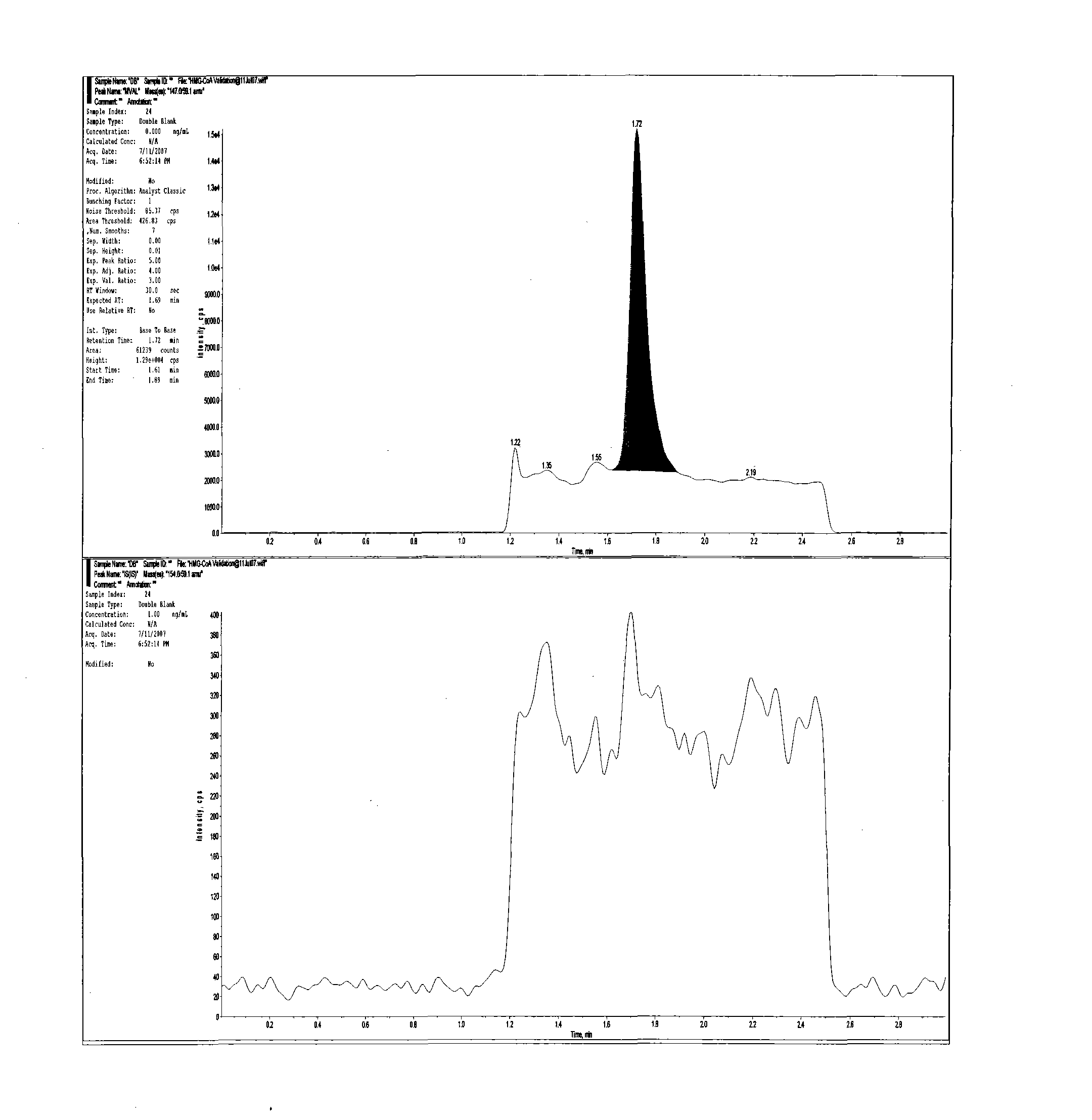
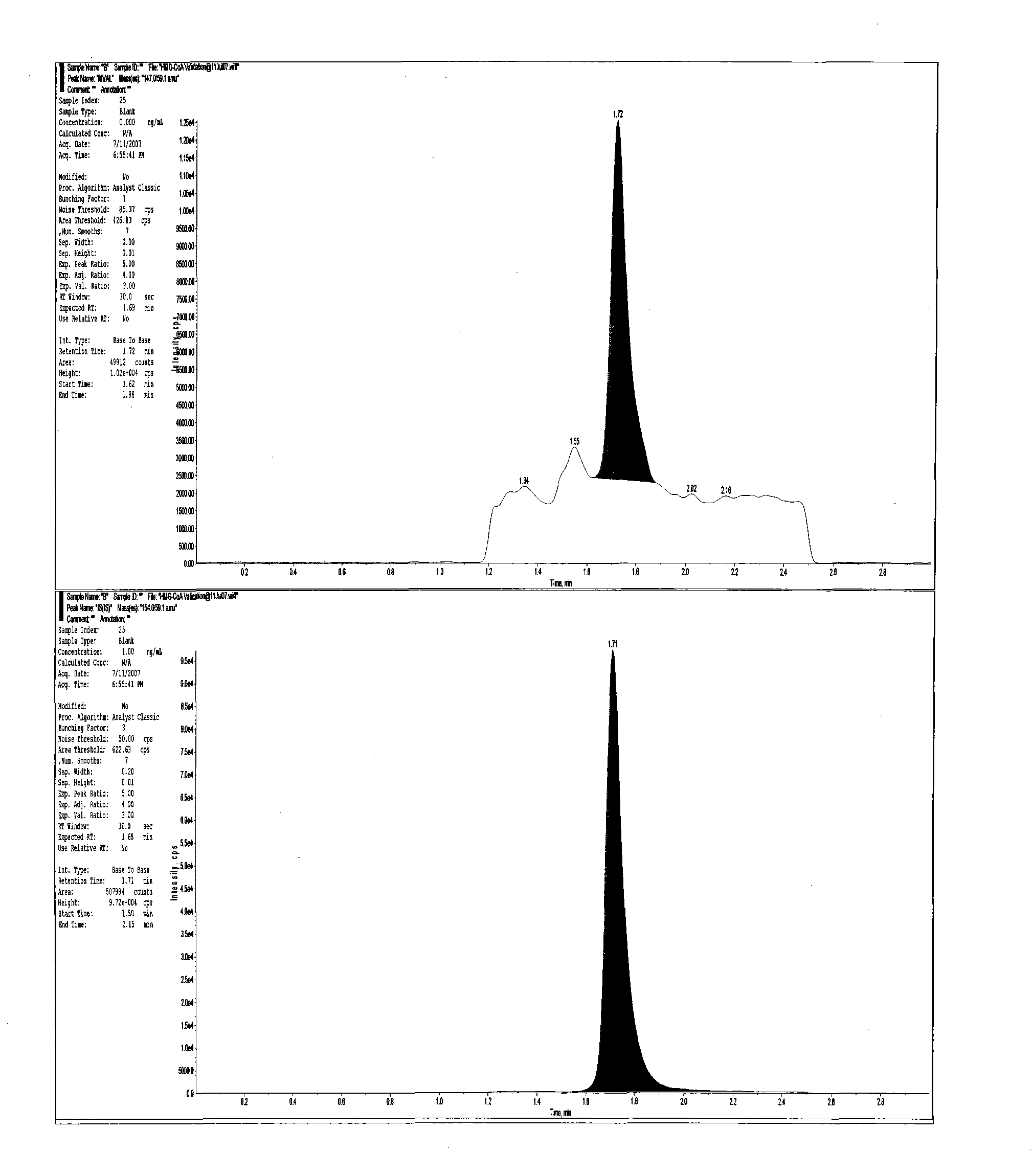
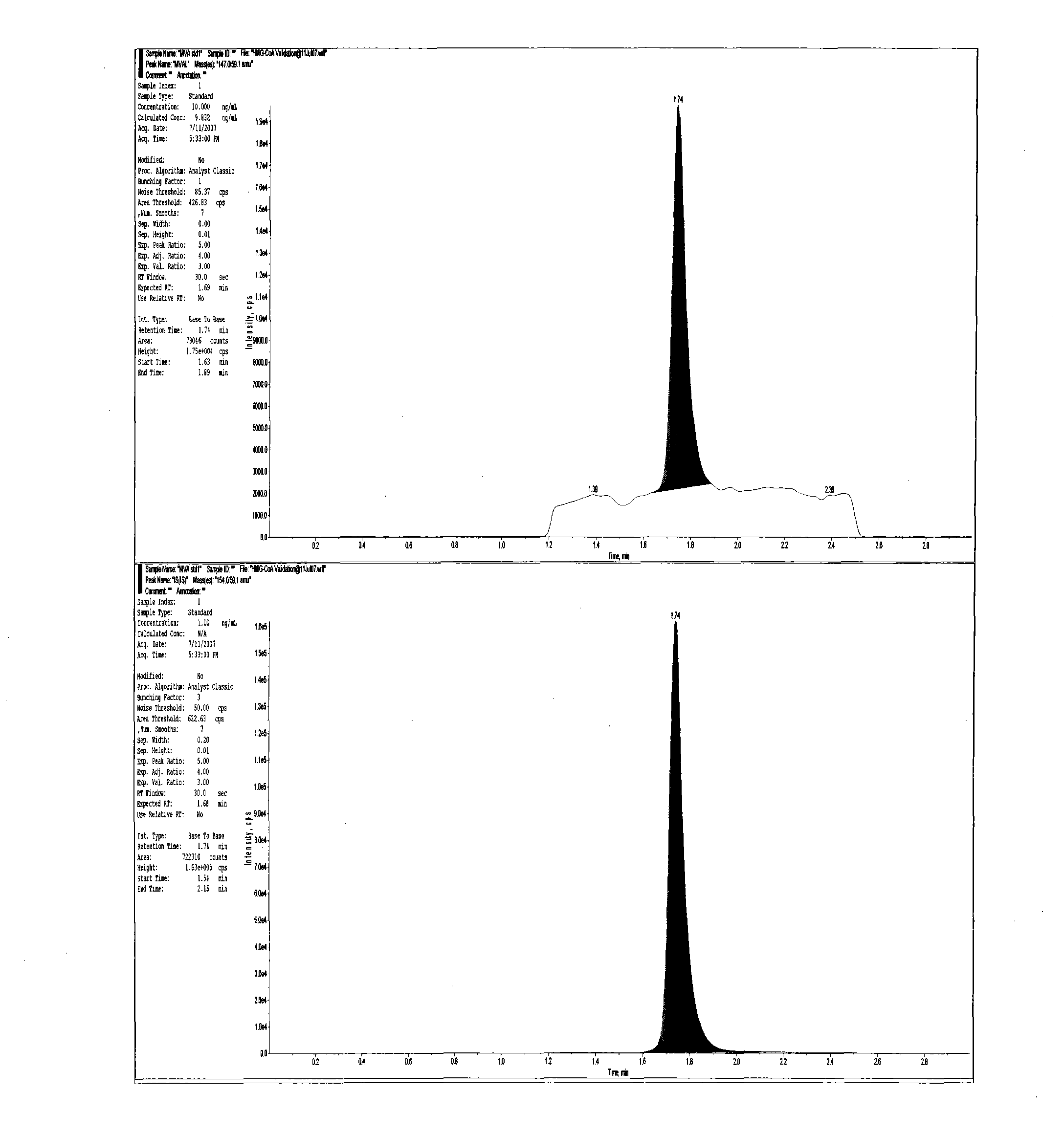
Method for realizing quantitative analysis on Lovastatin acid and HMG-CoA reductase inhibitor in human plasma
The invention discloses a method for realizing the quantitative analysis on Lovastatin acid and other HMG-CoA reductase inhibitors in human plasma by measuring HMG-CoA reductase inhibitor. By the confirmation of a test on the applicability of the method, when being used for testing the inhibitory activity of Lovastatin acid and other HMG-CoA reductase inhibitors in human plasma, the method can meet the requirements of methodology, so that the method can be applicable to bioresearches. All data can satisfy the acceptance standards of experiment design.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Red yeast prepn containing active components of glossy ganoderma and its prepn process
ActiveCN1636588AAdvanced technologySimple processOrganic active ingredientsMetabolism disorderFood additiveAdditive ingredient
The present invention provides one kind of red yeast preparation containing the active components of glossy ganoderma and its preparation process. The present invention is prepared with red yeast leavening containing the active components of glossy ganoderma and contains the main functional components including coarse polysaccharide not less than 4 mg each 100 g, total flavone not less than 3 mg each 100 g and lovastatin not less than 0.1 mg each 100 g. It may be prepared into oral liquid, soft capsule, powder, capsule, tablet, honeyed bolus, ointment, food additive and other preparation forms. The present invention includes many kinds of physiologically active matters, including polysaccharide, flavone, lovastatin, unsaturated fatty acid, protein, amino acid, etc. and has the synergistic blood fat reducing effect of red yeast and liver protecting effect of glossy ganoderma.
Owner:XIAN LIJUN PHARMA CO LTD
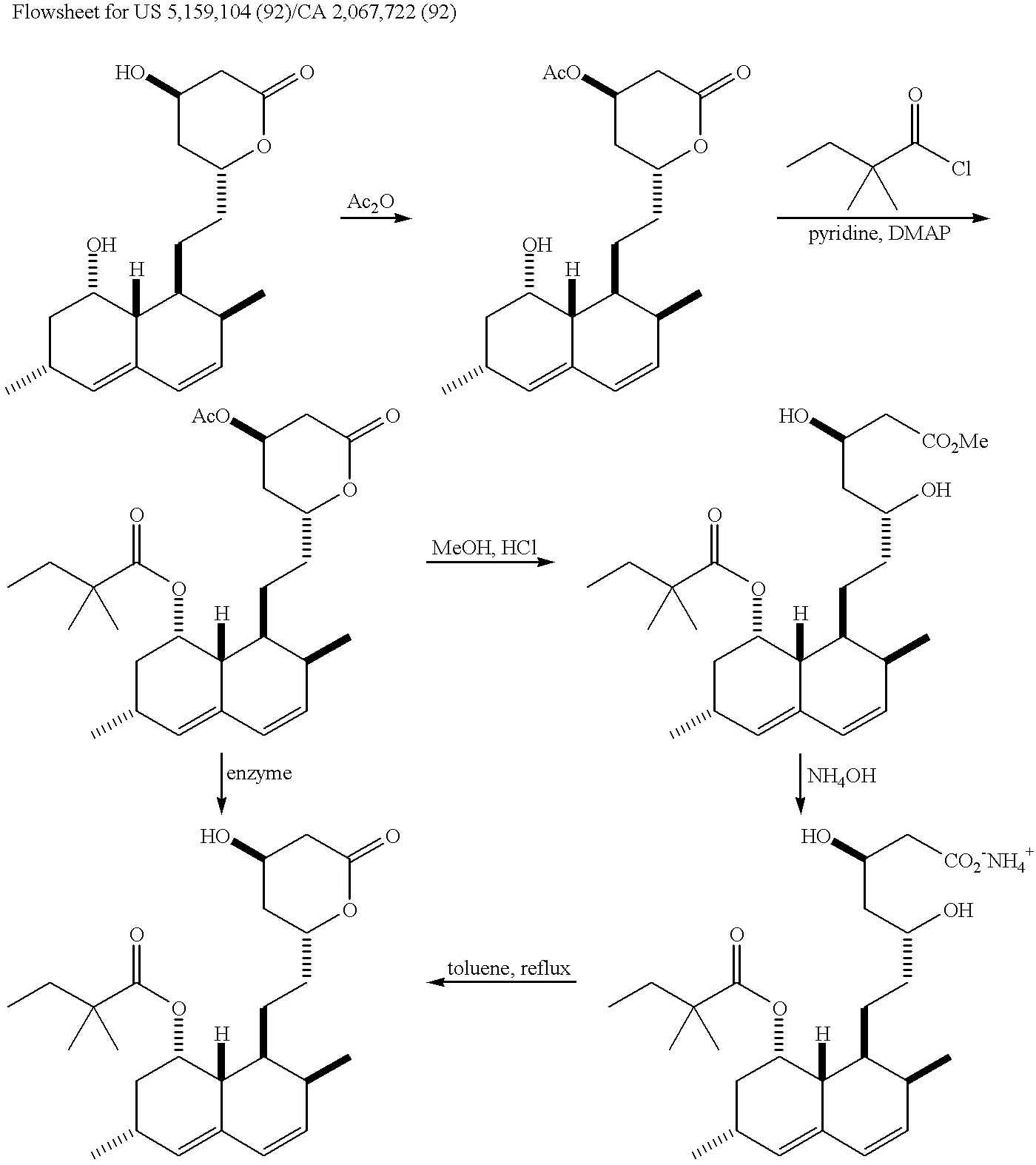
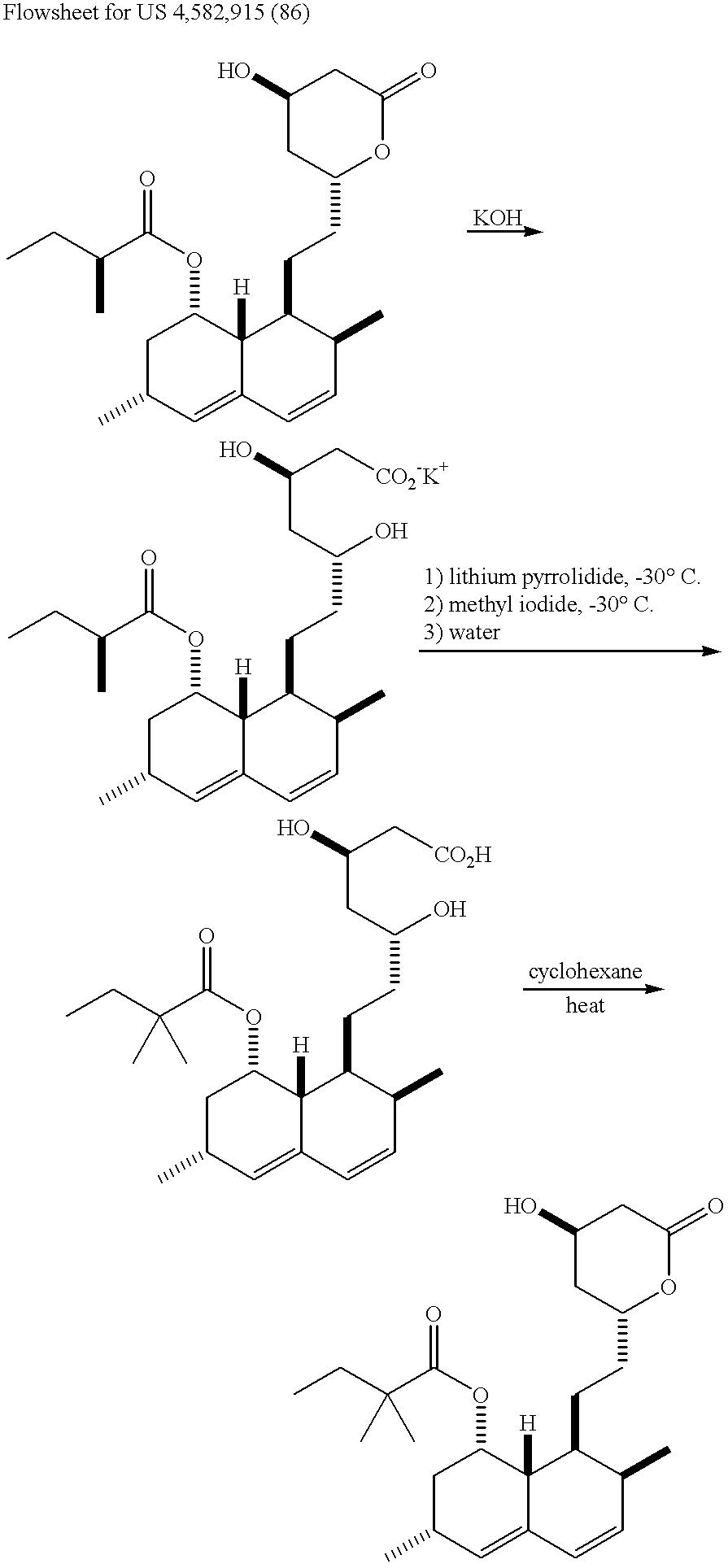
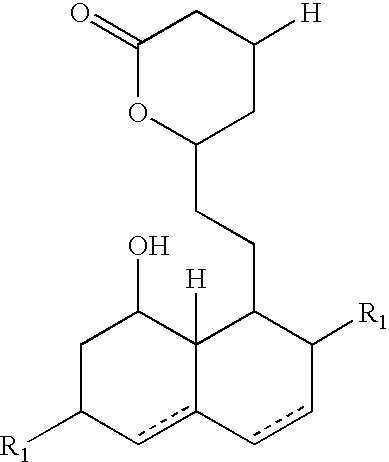
New salts of HMG-CoA reductase inhibitors
Lovastatin, pravastatin, simvastatin, mevastatin, atorvastatin, and derivatives and analogs thereof are known as HMG-CoA reductase inhibitors and are used as antihypercholesterolemic agents. The majority of them are produced by fermentation using microorganisms of different species identified as species belonging to Aspergillus, Monascus, Nocardia, Amycolatopsis, Mucor or Penicillium genus, some are obtained by treating the fermentation products using the methods of chemical synthesis or they are the products of total chemical synthesis. The present invention relates to the new amine salts of HMG-CoA reductase inhibitors, the preparation thereof, the preparation of pure HMG-CoA reductase inhibitors via amine salts thereof, use of the amine salts of HMG-CoA reductase inhibitors in the process for semisynthetic preparation of HMG-CoA reductase inhibitors, use of the amine salts of HMG-CoA reductase inhibitors in the process for biotechnological modification of HMG-CoA reductase inhibitors as well as the conversion of the amine salts of HMG-CoA reductase inhibitors into the pharmaceutically acceptable salts of the HMG-CoA reductase inhibitors and the conversion of the amine salts of HMG-CoA reductase inhibitors into the HMG-CoA reductase inhibitors in the lactone form.
Owner:LEK PHARMA D D
Production method of high-ring-opening-rate lovastatin
InactiveCN104726444AIncreased open loop rateImprove qualityMutant preparationMicroorganism based processesBiotechnologyMicroorganism
The invention belongs to the field of microbial technologies, and particularly relates to a production method of high-ring-opening-rate lovastatin. The method comprises the steps of mutagenizing monascus purpureus and fermenting the mutagenized strain. The method has the beneficial effects that according to the mutagenized strain and lovastatin production method disclosed by the invention, compared with conventional monascus production strains and production methods thereof, the ring opening rate of obtained lovastatin can be increased by over 1.5 times, and reach 68.9%, so that lovastatin products with higher quality are obtained.
Owner:山东中惠生物科技股份有限公司 +1
Composition beneficial to cardio-cerebrovascular
InactiveCN101347556APrevent obesityPrevent hyperlipidemiaMetabolism disorderFood preparationLovastatinCrataegus extract
The invention relates to a composition that is good for cardio-cerebral vessels, and the composition contains the components with following parts by weight: 10-200 parts of konjaku flour, 1-100 parts of hawthorn fruit extraction, 1-100 parts of tea extraction and 0.01-5 parts of red koji (count with Lovastatin), and the composition also contains barbary wolfberry fruit. The composition plays a positive role in preventing cardiovascular disease, intervenes in a plurality of formation phases of cardiovascular disease and can be used for preparing medicines, health-care food or food that can prevent and cure the cardiovascular disease.
Owner:毛晓敏
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com