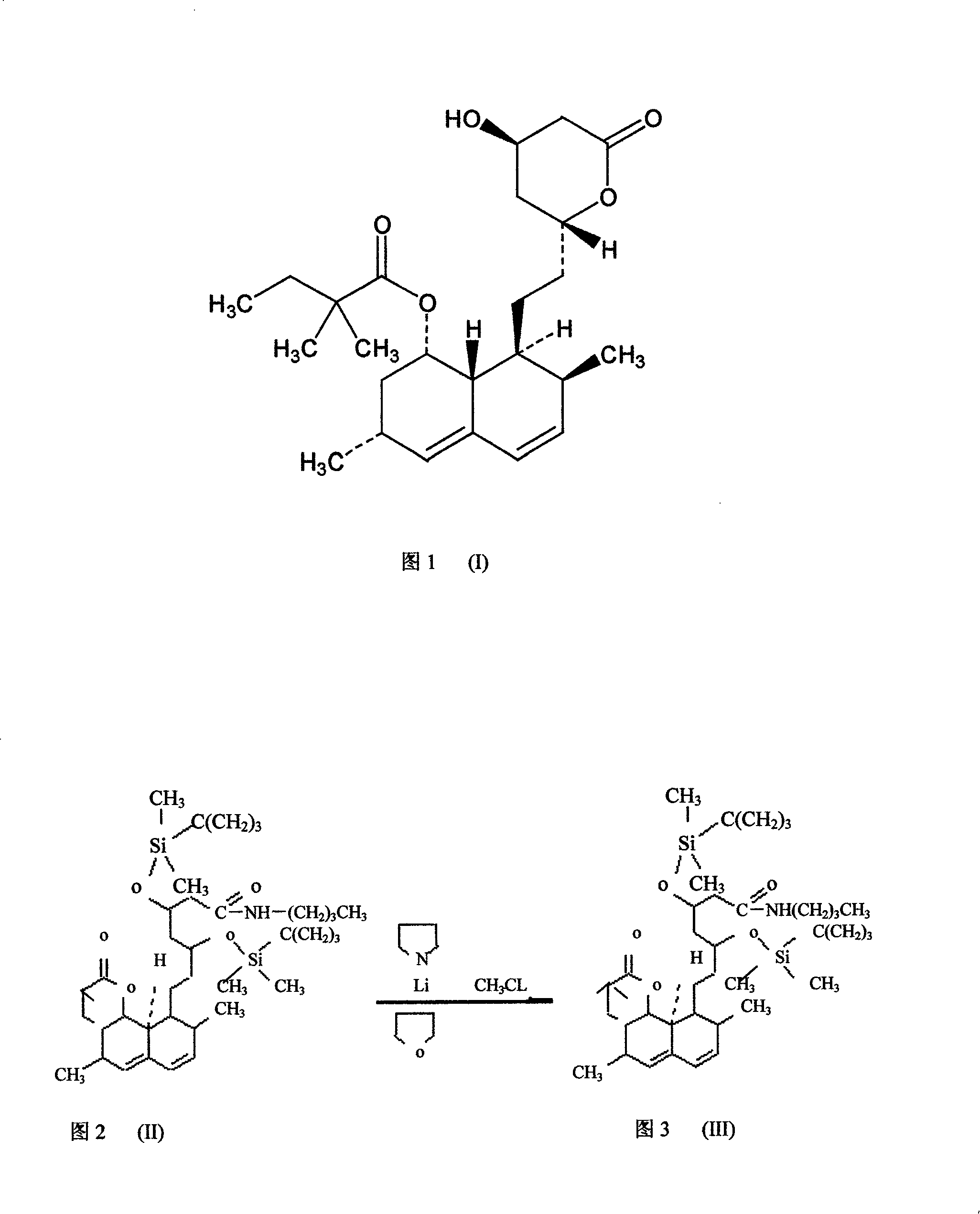
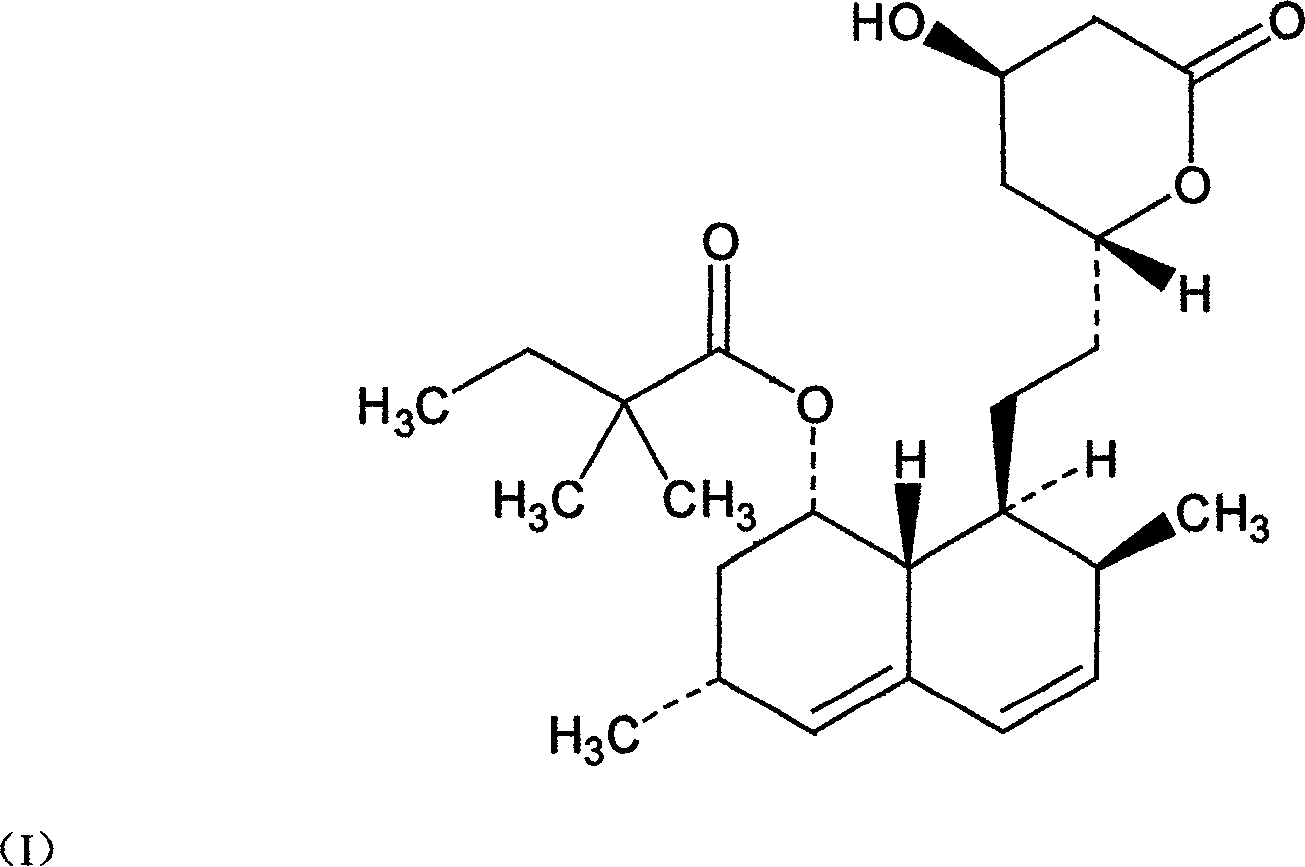
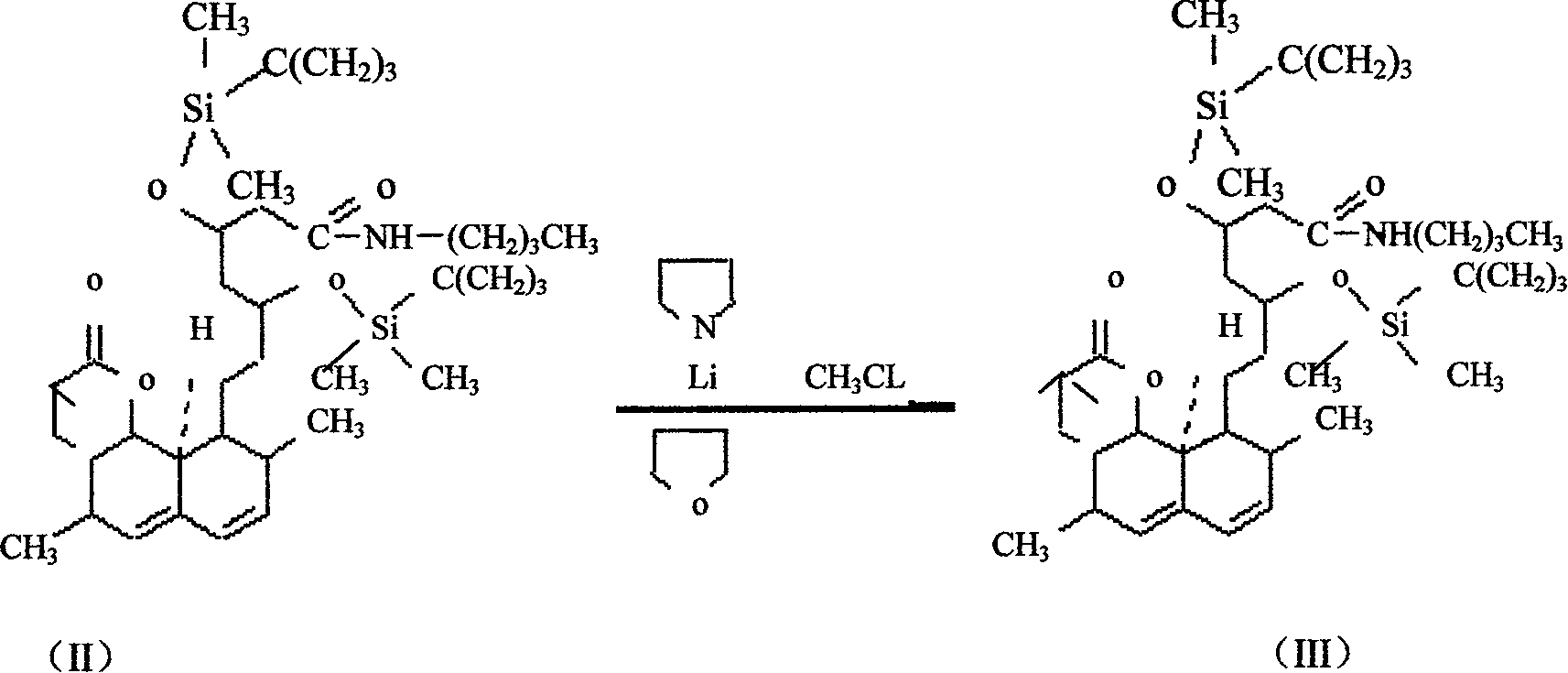
Method for synthesizing statins compounds
A technology of simvastatin and methylation, applied in the field of preparation of statin compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of lovastatin amide
[0029] Weigh 25Kg of lovastatin, and then add 29Kg of n-butylamine, then stir and slowly raise the temperature to 80°C, reflux for 1 hour, depressurize n-butylamine, and obtain a reddish-brown intermediate.
Embodiment 2
[0030] Embodiment 2: Preparation of lovastatin amide disiloxane
[0031] Add 100Kg of DMF to lovastatin amide, stir to dissolve, slowly add 35Kg of protective group tert-butyldimethylchlorosilane, after the heat release stops, then raise the temperature to 85°C and keep it for 3 hours, add water and cyclohexane to extract and discard The water layer and the cyclohexane layer were recovered under reduced pressure to obtain a reddish-brown viscous liquid.
Embodiment 3
[0032] Embodiment 3: the preparation of simvastatin amide disiloxane
[0033] Add tetrahydrofuran 95L\tetrahydropyrrole 125L, add n-butyllithium tetrahydrofuran solution 100L (about 20%) dropwise at -15°C--30°C, keep warm for 30 minutes after dropping, add tetrahydrofuran at -15°C--30°C 215L into the lovastatin amide disiloxane solution, keep warm for 1-4 hours, add dropwise methyl chloride tetrahydrofuran saturated solution at -10-0°C, control the end point by HPLC, add water to terminate the reaction, and concentrate the post-treatment oil layer under reduced pressure to obtain a red liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com