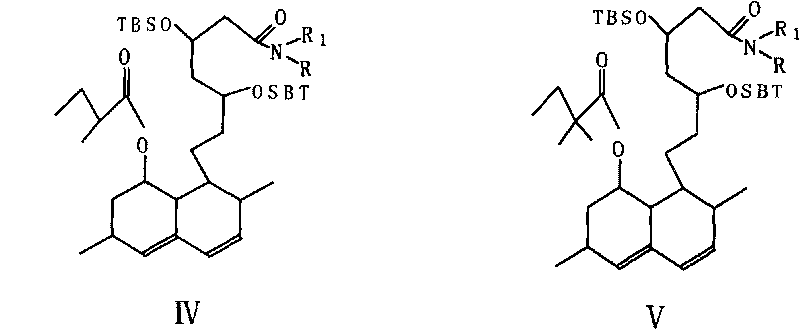Method for preparing simvastatin intermediate - simva-acylamide second silicon ether
A technology of simvastatin and simvastatin, which is applied in the directions of silicon organic compounds, organic chemistry, drug combination, etc., can solve the problems of increased preparation cost of simvastatin, difficult control of the production process, etc., so as to improve product purity and simplify production. Process, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
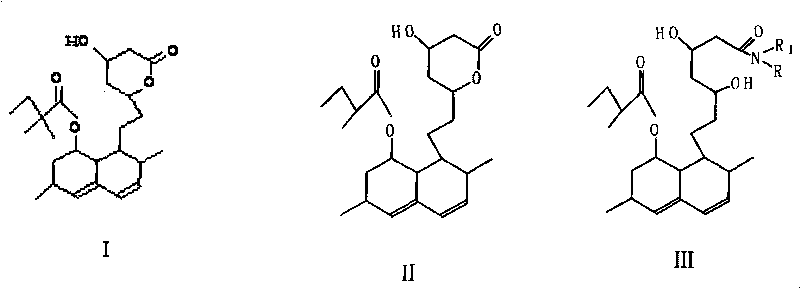
[0014] At room temperature, add 100g of lovastatin (II), add 80ml of n-butylamine, heat to 80°C under stirring, reflux for 1-1.5 hours, cool down to 50°C, evaporate excess n-butylamine under reduced pressure, and obtain lovastatin (III ) 118g, purity 98%. used directly in the next reaction.
[0015] The above-mentioned lovaamide (III) was dissolved in 250ml of DMF, 38g of imidazole and 88g of tert-butyldimethylsilyl chloride (TBSCl) were added, heated to 50°C, reacted for 3.5 hours, the mixture was cooled to room temperature, 1500ml of water was added, and 1800ml of cyclohexane Extract and separate the organic layer. After drying over anhydrous sodium sulfate, it was concentrated to dryness under reduced pressure to obtain lovaamide disiloxane (IV) with a purity of over 97%, which was directly used in the next reaction.
[0016] Under the protection of nitrogen, cool the mixture of 50ml of pyrrolidine and 300ml of tetrahydrofuran dried through molecular sieves to -25°C, add ...
Embodiment 2
[0020] According to Example 1, lovaamide, lovaamide disiloxane and lithium pyrrolidinyl were prepared.
[0021] Compound (IV), add 650ml of tetrahydrofuran, stir to dissolve, cool to -45°C, under the protection of nitrogen, add pyrrolidinyl lithium solution dropwise at -40°C ~ -45°C, after dropping, keep warm for 2.5 hours, then drop Dimethyl sulfate was added, and the end point was controlled by HPLC. Water was added to terminate the reaction, and after treatment, the organic layer was concentrated to dryness under reduced pressure to obtain the product (V). Unreacted raw material (IV) 0.48%.
Embodiment 3
[0023] According to Example 1, lovaamide, lovaamide disiloxane and lithium pyrrolidinyl were prepared.
[0024] Compound (IV), add 350ml tetrahydrofuran and 350ml cyclohexane, dissolve under stirring, cool to -35°C, under vigorous stirring, add the prepared pyrrolidinyl lithium solution at -35°C ~ -30°C, and complete the addition. Heat preservation reaction for 2 hours, then add 60ml of methyl p-toluenesulfonate, the temperature rises, then cool to -30°C, stir for 1 hour, add 500ml of water, stir for 10 minutes, let stand, separate the organic layer, wash and concentrate under reduced pressure to After drying, simvaxamide disiloxane (V) and 0.51% of unreacted raw material lovaamide disiloxane (IV) were obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com