Manufacturing process, such as three dimensional printing, including binding of water-soluble material followed by softening and flowing and forming films of organic-solvent-soluble material
a three-dimensional printing and manufacturing process technology, applied in the field of biostructure construction, can solve the problems of increasing the difficulty of dispense of organic solvents such as chloroform from the printhead than water or aqueous solutions, exhibiting further difficulties, and unusually large tendency to spread
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
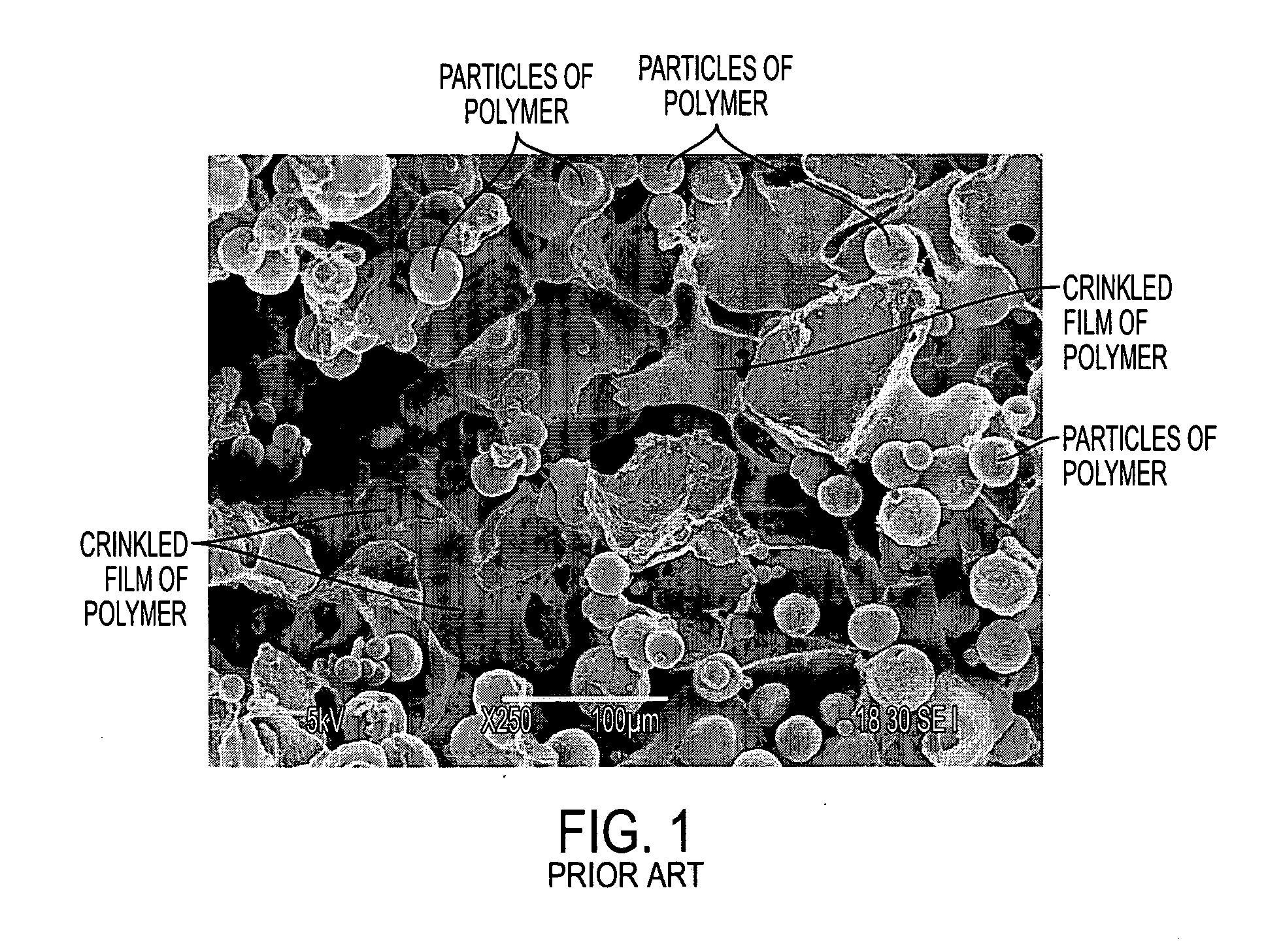
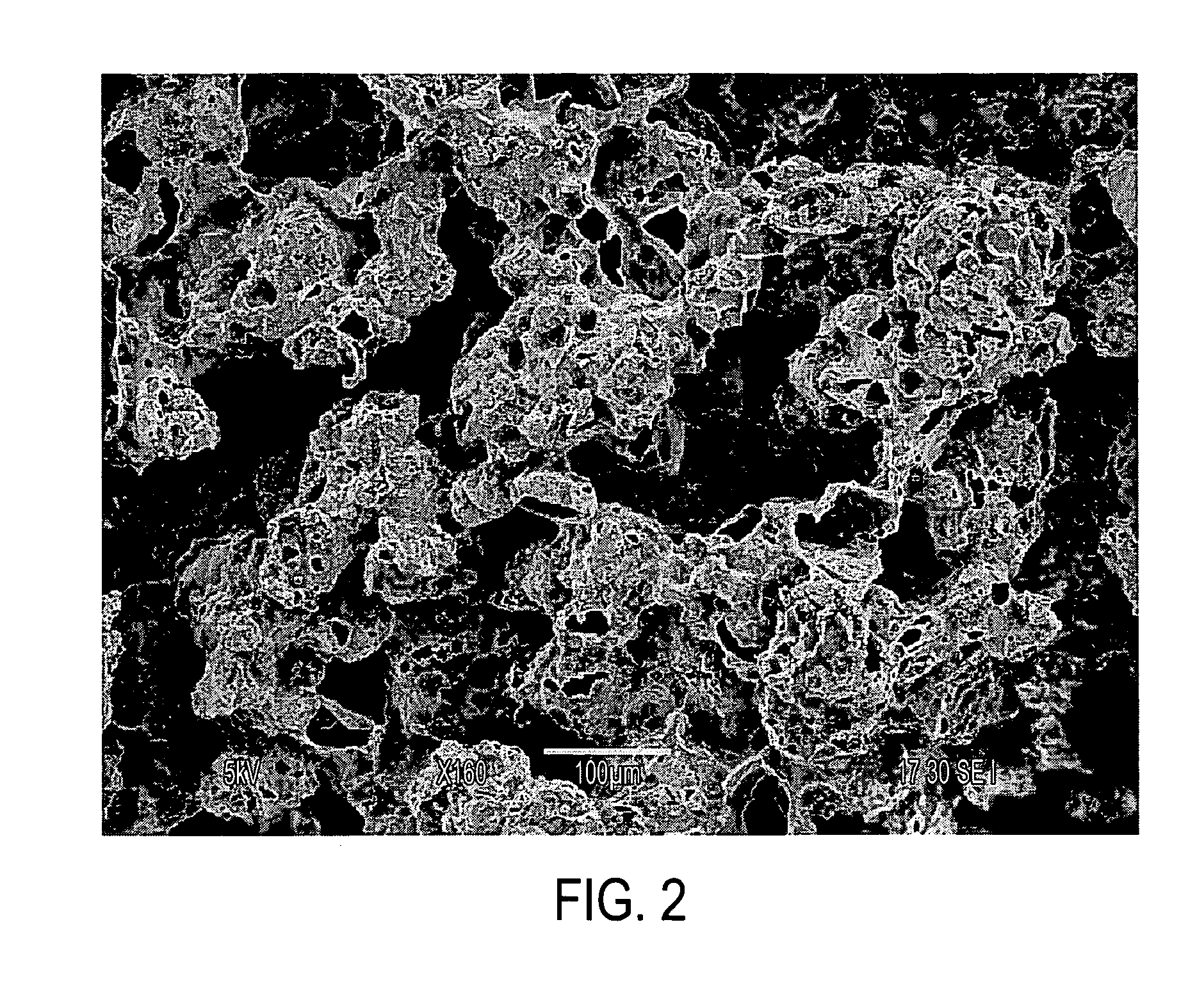
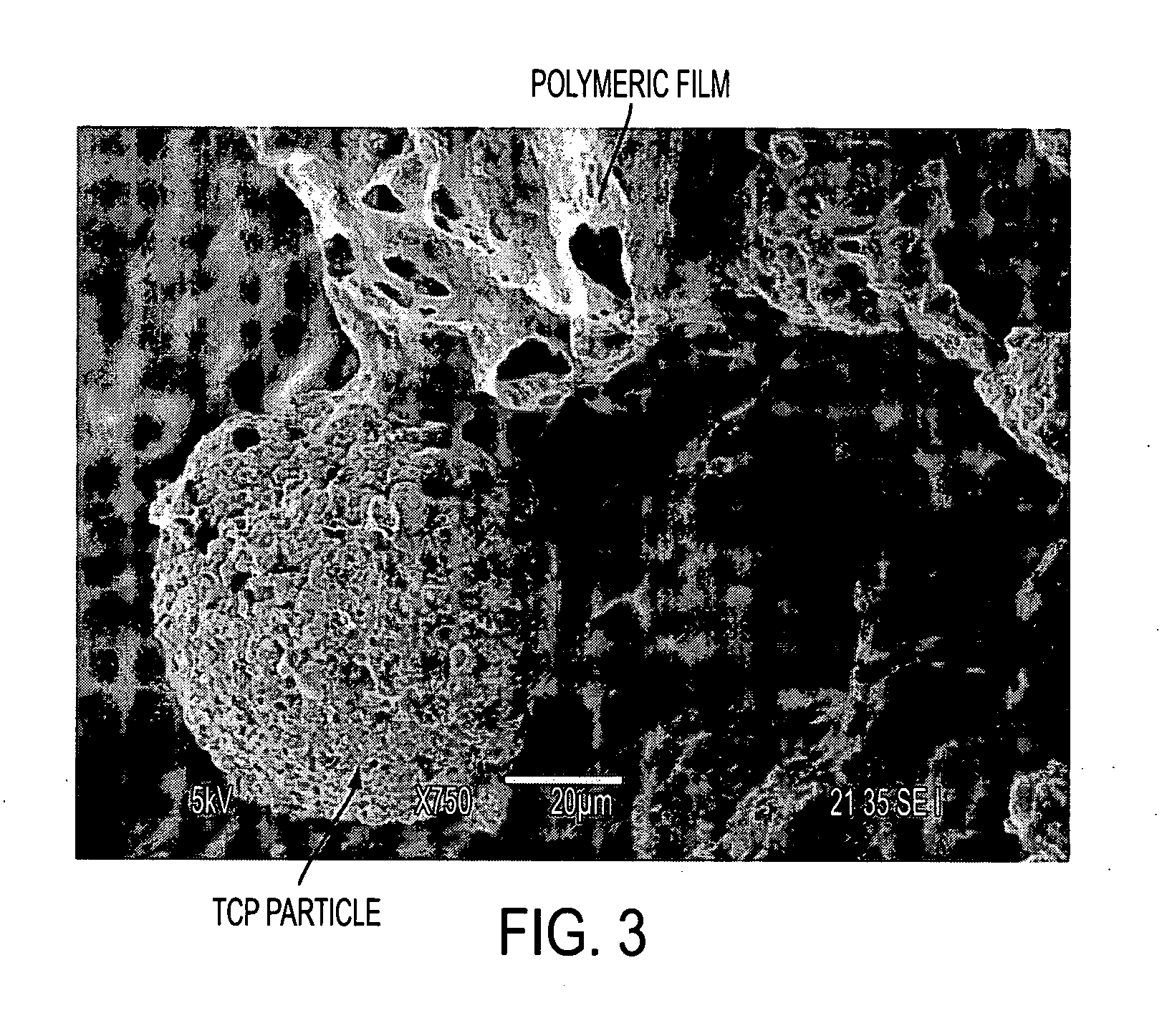
[0109] This Example compares polymer structures which were 3D-printed using the water printing followed by exposure to solvent vapor, against polymer structures which were 3D-printed using conventional dispensing of liquid chloroform onto a powder bed operating using the dissolution / resolidification mechanism. Both powder beds contained a water-soluble porogen for later leaching out as an aid to creating porosity in the finished biostructure. Specifically, this Example compares the microstructures of those two types of samples, as already presented in FIG. 1 and FIG. 2.
[0110] First of all, FIG. 1 illustrates the microstructure of the structure made by conventional 3DP with dispensed liquid chloroform. The powder used in this case was 80:20 NaCl:PCL. The powder mixture did not include any substantially-insoluble material.
[0111] What can be observed is that there is first of all some basic polymeric structure, which has the form of a film which is irregularly shaped. This basic poly...
example 2
[0116] This example also compares polymer structures repeating essentially the same two printing methods and conditions as were presented and compared in Example 1. However, what is presented in this Example is the macrostructure, rather than microstructure. Two comparisons are presented in this Example.
[0117]FIG. 8 shows a face or top view of two structures. The two articles shown in FIG. 8 are both related to the structure shown in FIG. 4a, but they are not exactly identical to each other, since they come from different sub-layers of that structure. Nevertheless, the two articles are of a similar nature and in particular the size scales of the geometric features in the two samples are essentially the same, and so there is validity in comparing the fuzziness or sharpness of the two structures. Again, in this case, the powder mixture did not include any substantially-insoluble material. For this comparison, chloroform printing had to be done with masks and water printing was done w...
example 3
[0119] This example compares printing onto the same powder bed composition with a binder liquid which was pure water and printing with a binder liquid which is a solution of sucrose in water. FIG. 10 shows a macroscopic grid pattern made by each of these two methods. In FIG. 10, the illustration on the left shows a structure formed by printing with a sucrose-water solution, and the illustration on the right shows a structure formed by printing with pure water. In each case, the composition of the powder mixture was 80% sucrose, 20% polycaprolactone. The powder mixture did not include any substantially-insoluble material. The saturation parameter used during printing was about 15% (i.e., volume of dispensed liquid compared to empty volume in the voxel). These photographs are of the preform only, prior to any exposure to solvent vapor or dissolution of the water-soluble structure. It appears that the structure resulting from the printing with the sucrose solution is better held togeth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com