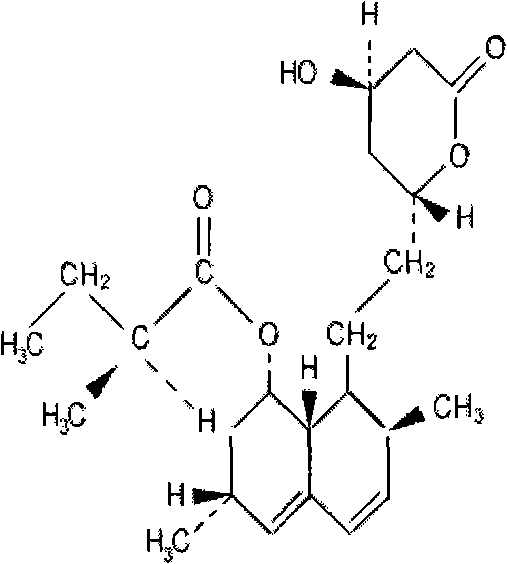
Method for producing lovastatin by fermentation and fermentation medium used by same
A fermentation medium and lovastatin technology, which is applied in the field of biopharmaceuticals, can solve the problems of low lovastatin content, high raw material cost, long fermentation cycle and the like, and achieve the effects of shortening the fermentation cycle, reducing the cost of raw materials and reducing the production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] In this embodiment, 5M 3 The fermentation tank is taken as an example to illustrate the process of preparing lovastatin by fermentation of the present invention.
[0083] The formula of the fermentation medium by weight / volume percentage is: 20.0% maltose, 4.0% cottonseed powder, 1.0% peptone, 0.5% solid malt extract, 0.2% sodium chloride, 0.05% potassium dihydrogen phosphate, 0.05% magnesium sulfate, defoaming Agent 0.05%, adjust the pH to 6.5.
[0084] The preparation method of fermentation medium is as follows:
[0085] 1. Add an appropriate amount of drinking water to the batching tank, then put in the raw materials (except maltose) of the formula amount, stir evenly, and pump to 5M 3 In the fermenter, the volume was adjusted to a certain volume with drinking water, and sterilized at 121°C for 30 minutes.
[0086] 2. Add an appropriate amount of drinking water to the batching tank, then put in the formula amount of maltose, stir evenly, pump it into the sugar tan...
Embodiment 2
[0090] In this embodiment, 5M 3 The fermentation tank is taken as an example to illustrate the process of preparing lovastatin by fermentation of the present invention.
[0091] The formula of the fermentation medium is: maltose 18.0%, cottonseed powder 4.0%, peptone 1.0%, solid malt extract 0.5%, sodium chloride 0.2%, potassium dihydrogen phosphate 0.05%, magnesium sulfate 0.05%, adjust pH is 6.5.
[0092] The preparation method of fermentation medium is as follows:
[0093] 1. Add an appropriate amount of drinking water to the batching tank, then put in the raw materials (except maltose) of the formula amount, stir evenly, and pump to 5M 3 In the fermenter, the volume was adjusted to a certain volume with drinking water, and sterilized at 121°C for 30 minutes.
[0094] 2. Add an appropriate amount of drinking water to the batching tank, then put in the formula amount of maltose, stir evenly, pump it into the sugar tank, use drinking water to set the volume to a certain vo...
Embodiment 3
[0098] In this embodiment, 50M 3 The fermentation tank is taken as an example to illustrate the process of preparing lovastatin by fermentation of the present invention.
[0099] The formula of the fermentation medium by weight / volume percentage is: 20.0% maltose, 4.0% cottonseed powder, 1.0% peptone, 0.5% solid malt extract, 0.2% sodium chloride, 0.05% potassium dihydrogen phosphate, 0.05% magnesium sulfate, defoaming Agent 0.05%, adjust the pH to 6.5.
[0100] The preparation method of fermentation medium is as follows:
[0101] 1. Add an appropriate amount of drinking water to the batching tank, then put in the raw materials (except maltose) of the formula amount, stir evenly, and pump to 50M 3 In the fermenter, the volume was adjusted to a certain volume with drinking water, and sterilized at 121°C for 30 minutes.
[0102]2. Add an appropriate amount of drinking water to the batching tank, then put in the formula amount of maltose, stir evenly, pump it into the sugar ta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com