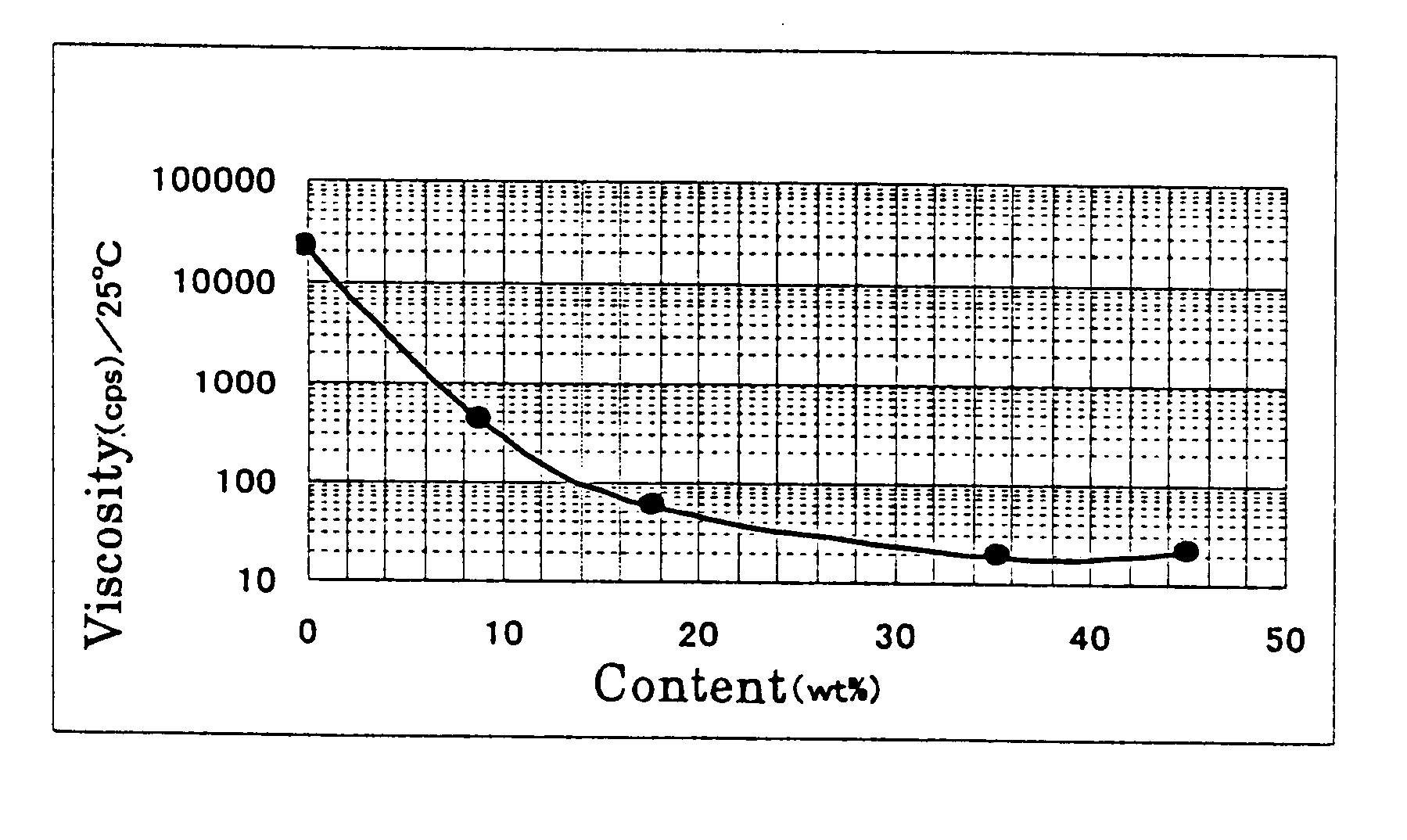
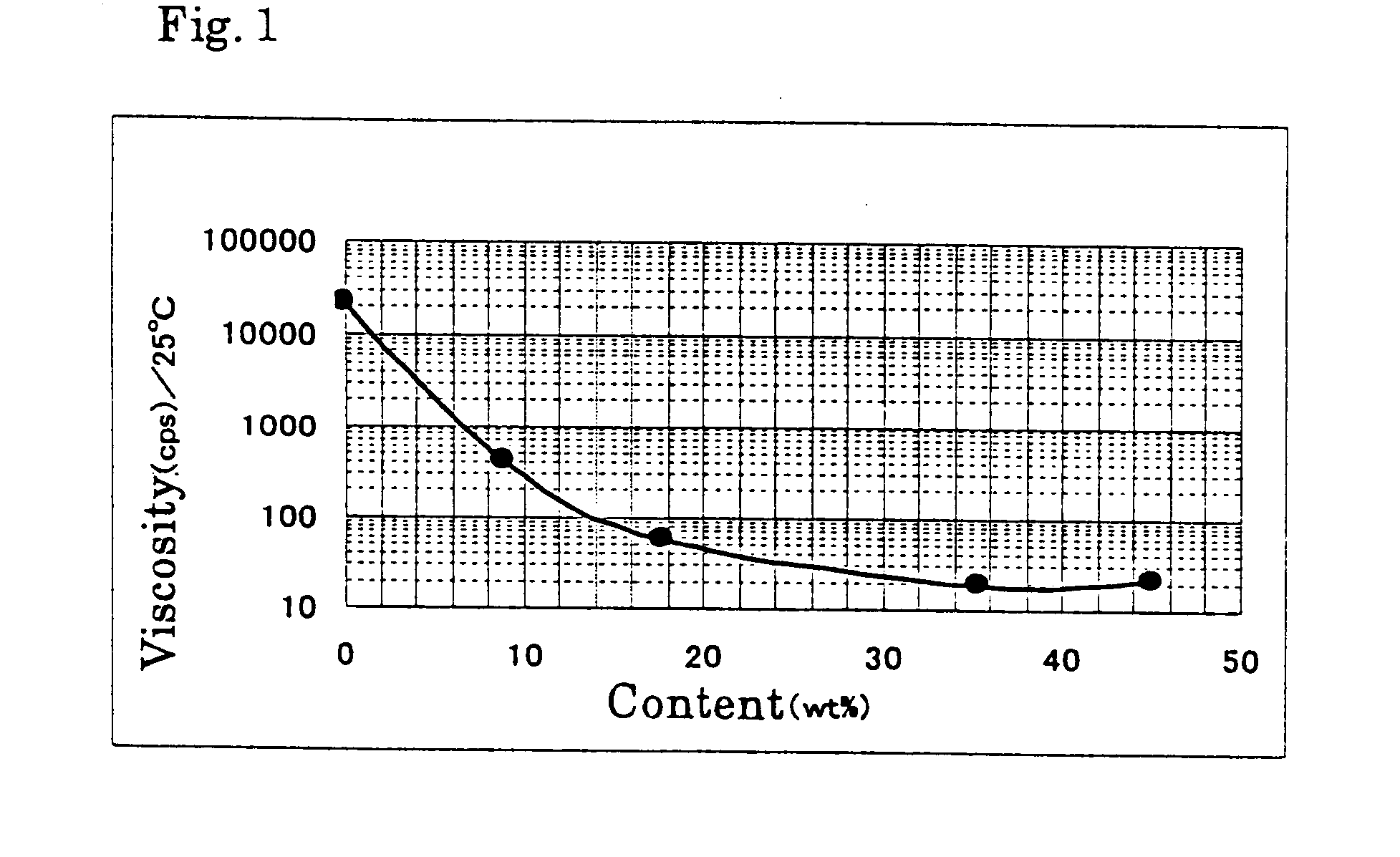
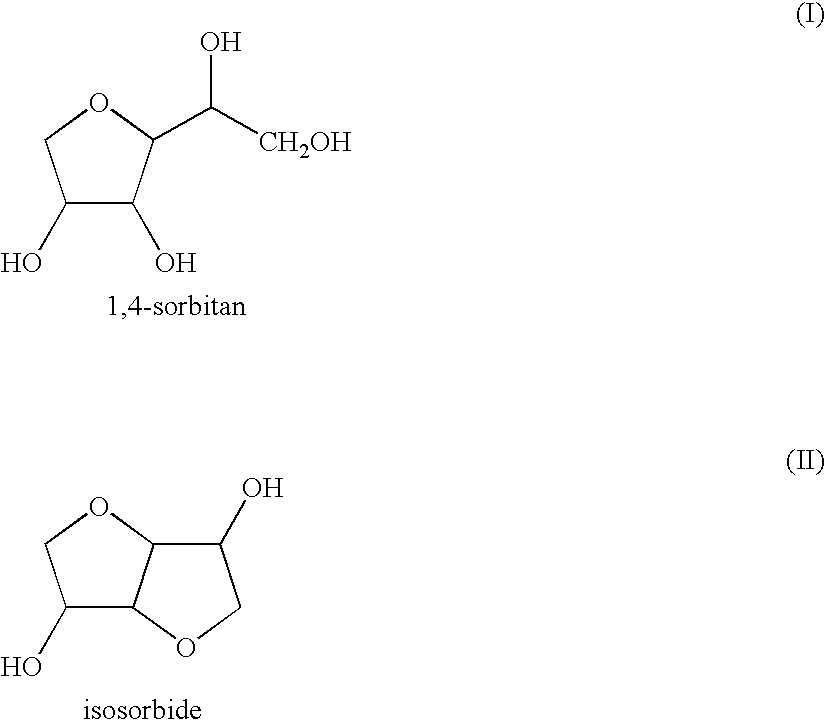
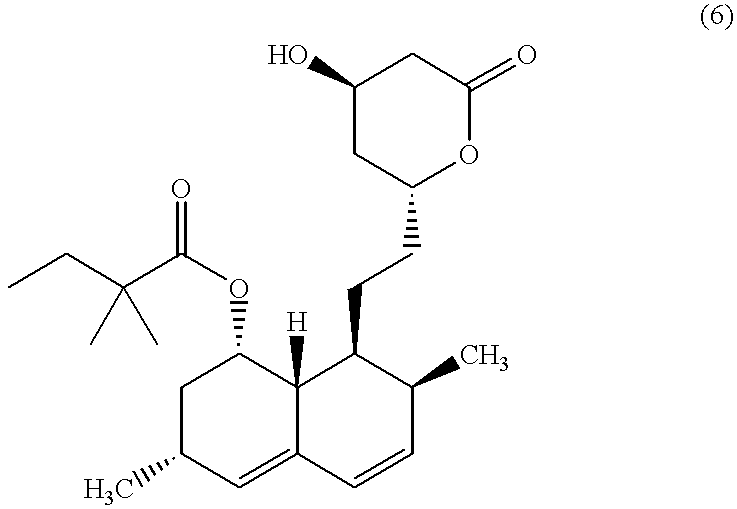
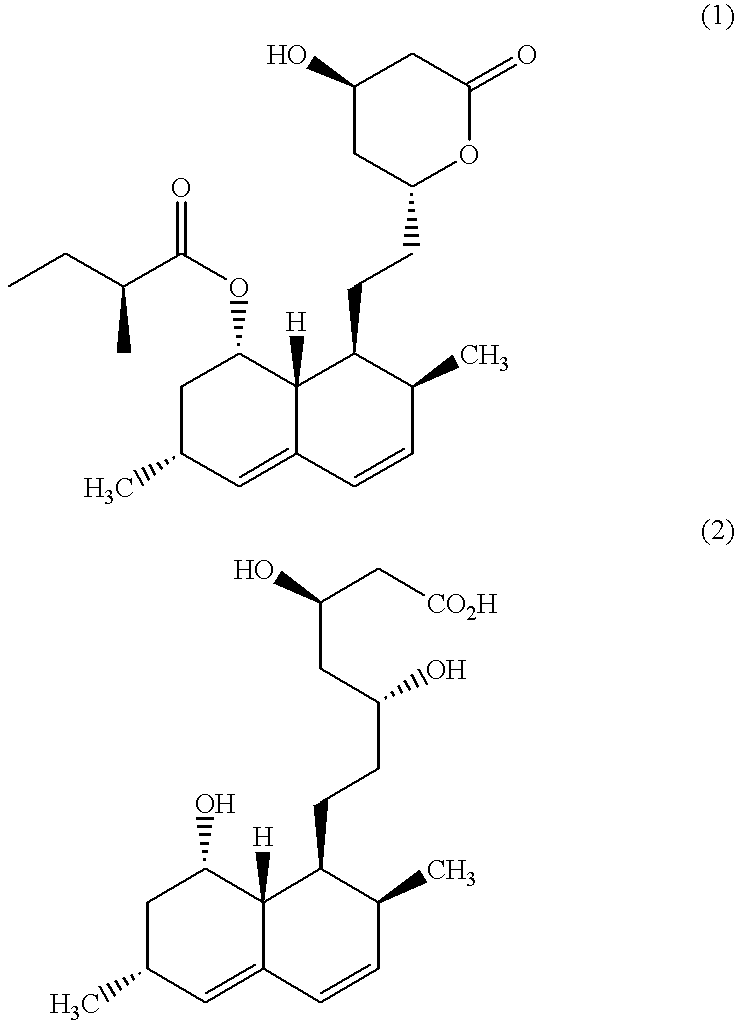
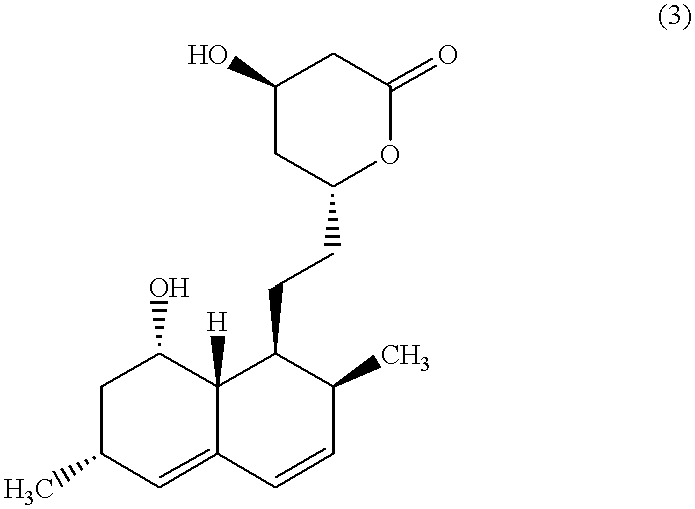
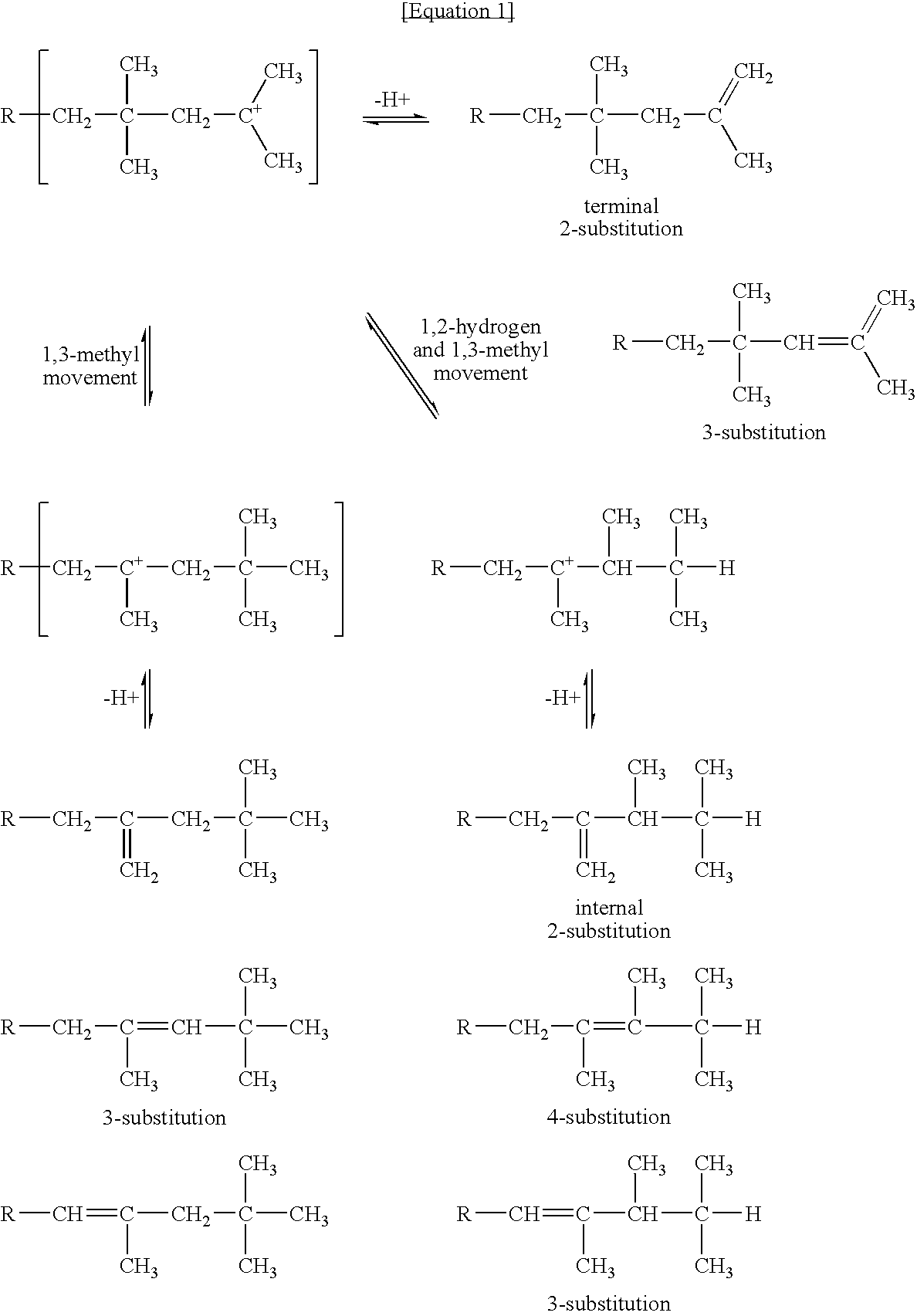
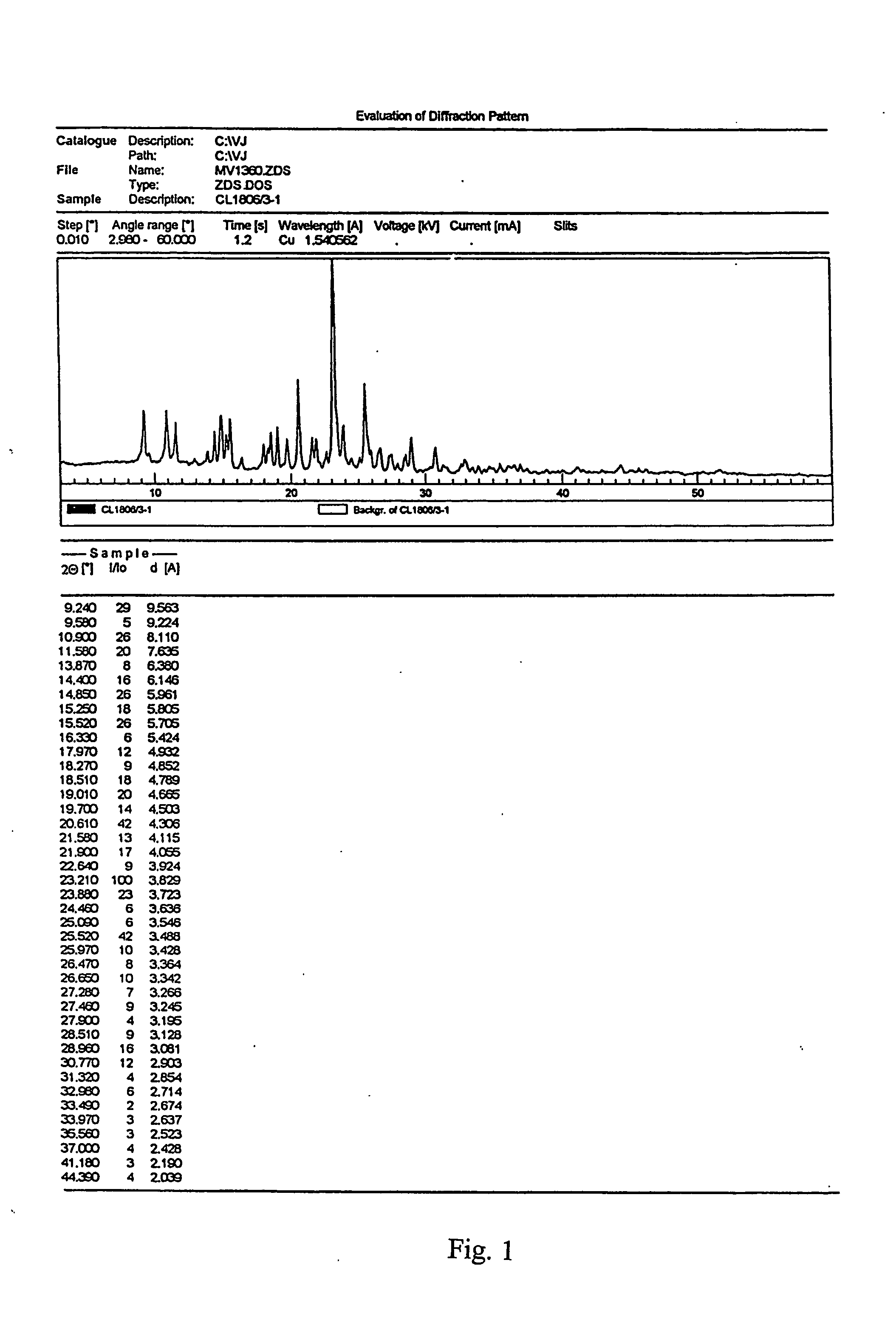
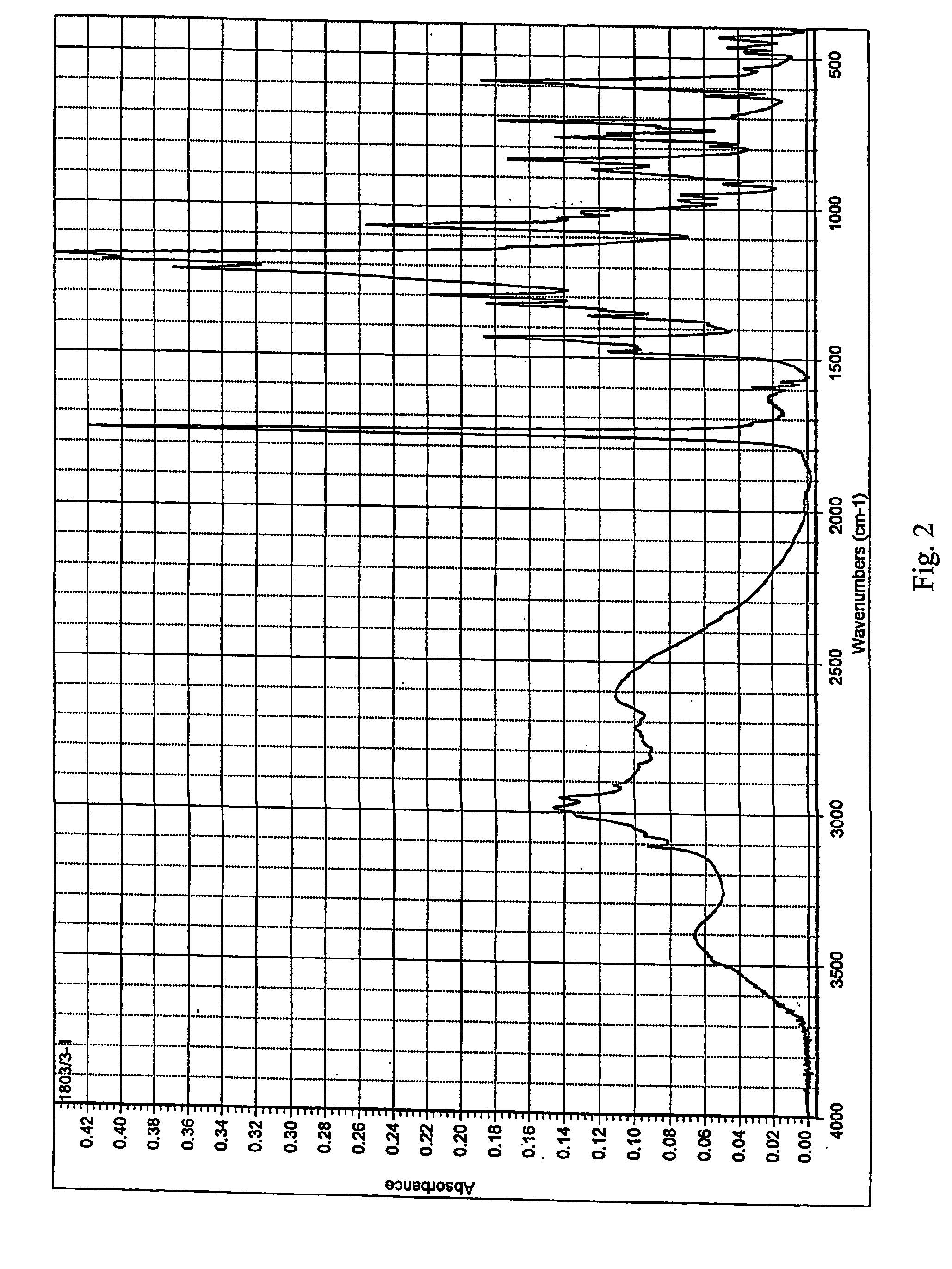
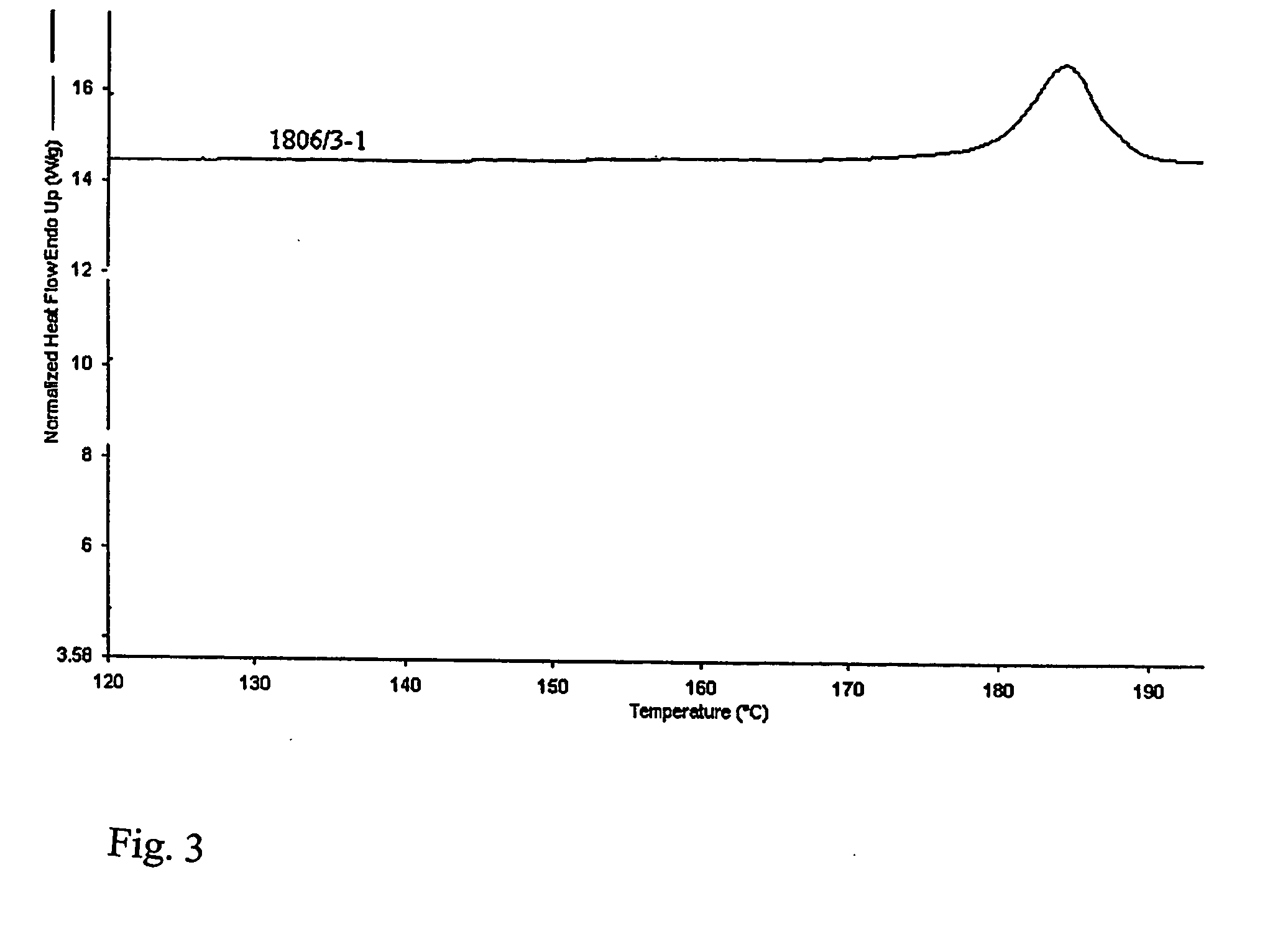
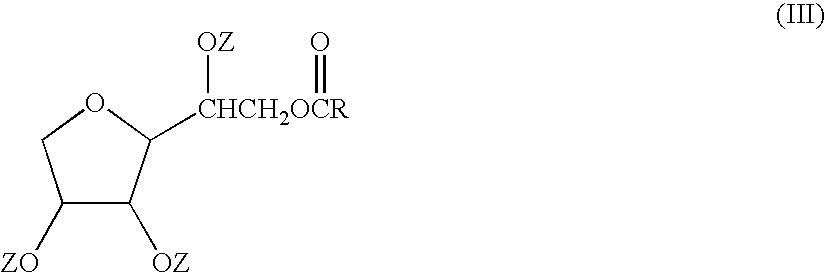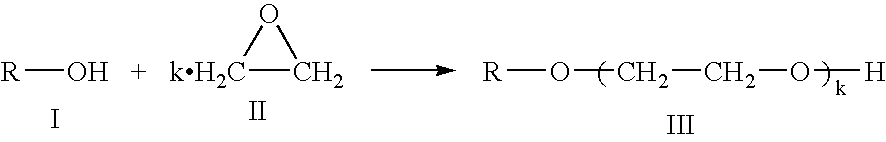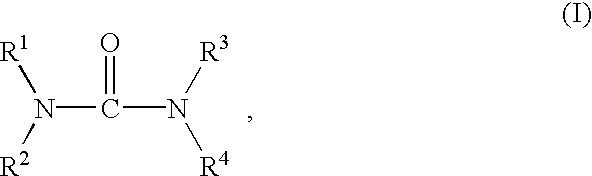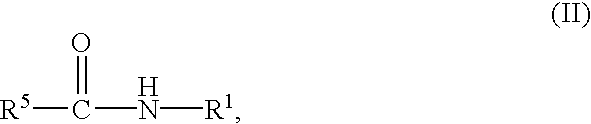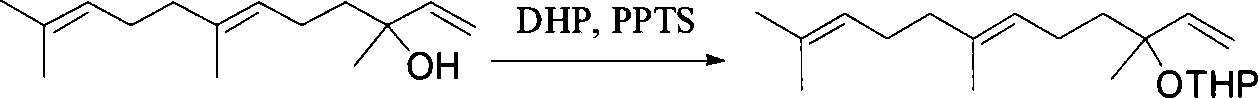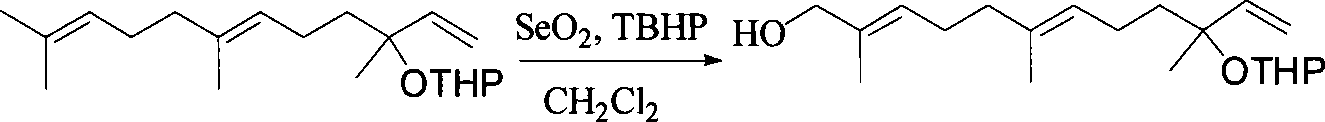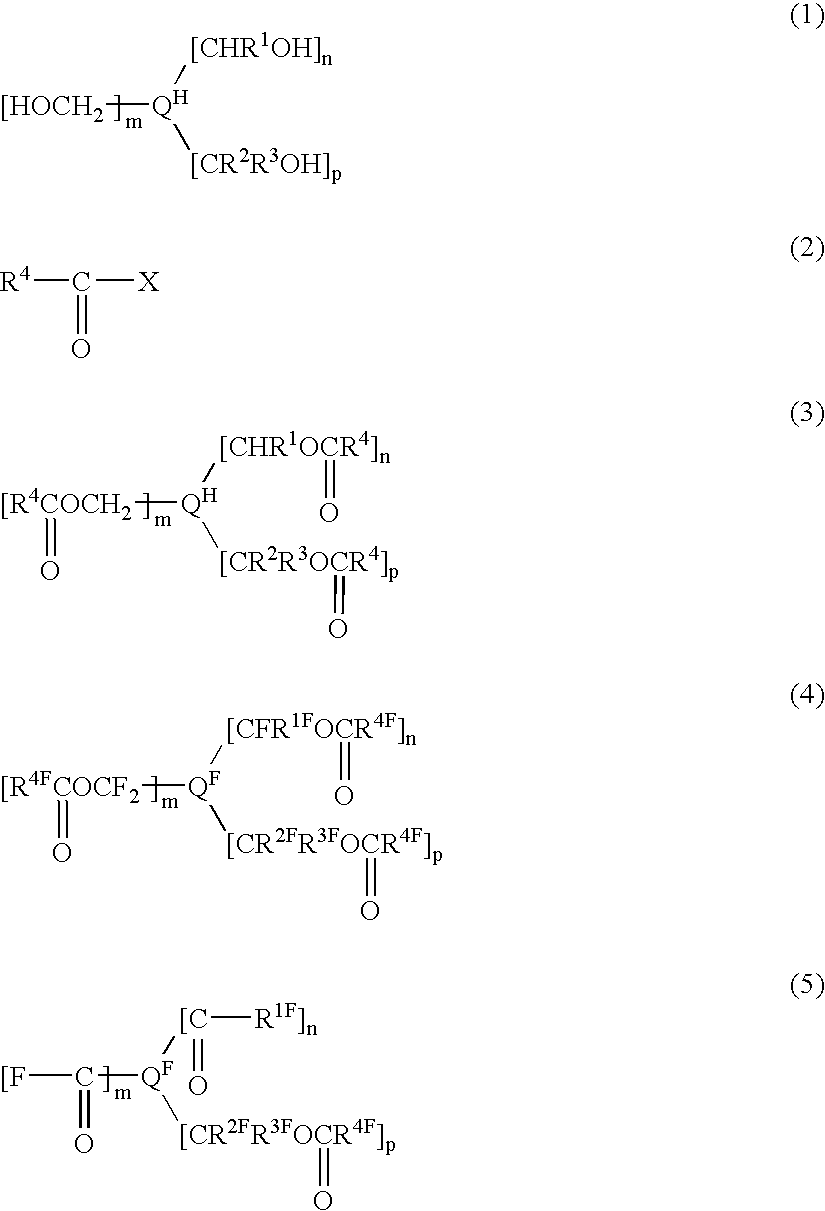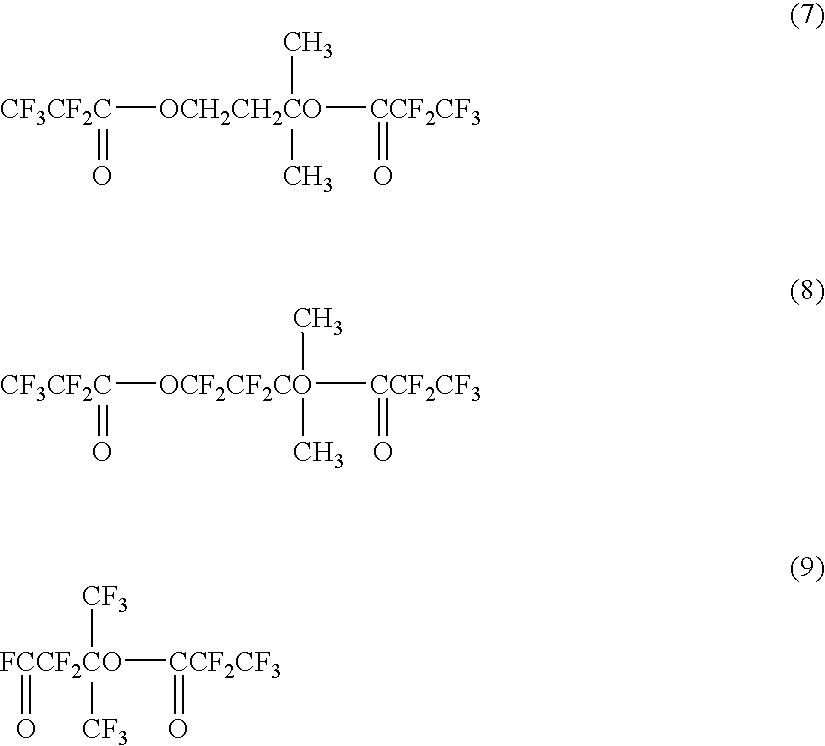Patents
Literature
150 results about "Tertiary alcohols" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
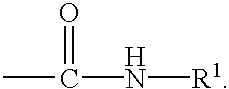
In alcohol: Structure and classification of alcohols Similarly, a tertiary alcohol has the hydroxyl group on a tertiary (3°) carbon atom, which is bonded to three other carbons.
Method for preparing N-long chain acyl neutral amino acid
InactiveUS6703517B2Efficient conductionLow viscosityCosmetic preparationsHair cosmeticsNeutral Amino AcidsAlanine
A method for preparing a highly purified N-long chain acyl neutral amino acid for use of a detergent and the like in a simple and convenient manner and in a high yield by reacting a neutral amino acid such as glycine, gamma-aminobutyric acid, and alanine, with a saturated or unsaturated fatty acid halide having 8 to 22 carbon atoms, wherein the reaction is performed in a mixture of water and one or more kinds of hydrophilic organic solvents selected from the group consisting of acetone, acetonitrile, a secondary alcohol having 3 or 4 carbon atoms, and a tertiary alcohol having 4 carbon atoms such as isopropanol, sec-butanol, and tert-butanol in the presence of a base.
Owner:AJINOMOTO CO INC
Biofuel Composition and Method of Producing a Biofuel
InactiveUS20100037513A1Reduce needBiofuelsLiquid carbonaceous fuelsHexadecaneSimple Organic Compounds
An emulsified biofuel composition comprising: (A) a continuous phase comprising about 50-95 wt % of at least one liquid oil of vegetable or animal origin or mixtures thereof; (B) a water-containing dispersed phase comprising about 1-50 wt % water; (C) about 1-25 wt % of hydroxyl-containing organic compound selected from the group consisting of mono-, di-, tri- and polyhydric alcohols, provided that when a monohydric alcohol is used there is also present at least one of tert-butyl alcohol, at least one C2-C4 alkylene glycol or a mixture of both; (D) about 0.05-10 wt % of at least one emulsifier; wherein the dispersed water-containing droplets have an average particle size of less than about 20 microns. The biofuel is prepared from these components by mixing under high shear conditions, preferably with ultrasonic energy. The emulsifier(s) preferably exhibit a hydrophilic-lipophilic balance of about 8.5 to about 18 and the biofuel includes a cetane enhancer and mixture of an alcohol and mono- or poly-alkylene glycol.
Owner:NEW GENERATION BIOFUELS +1
Apparatus and method for producing plane-parallel flakes
InactiveUS6270840B1Increase productionLow production costTransportation and packagingPigment preparation by PVD/CVD methodsAlcoholFluoride
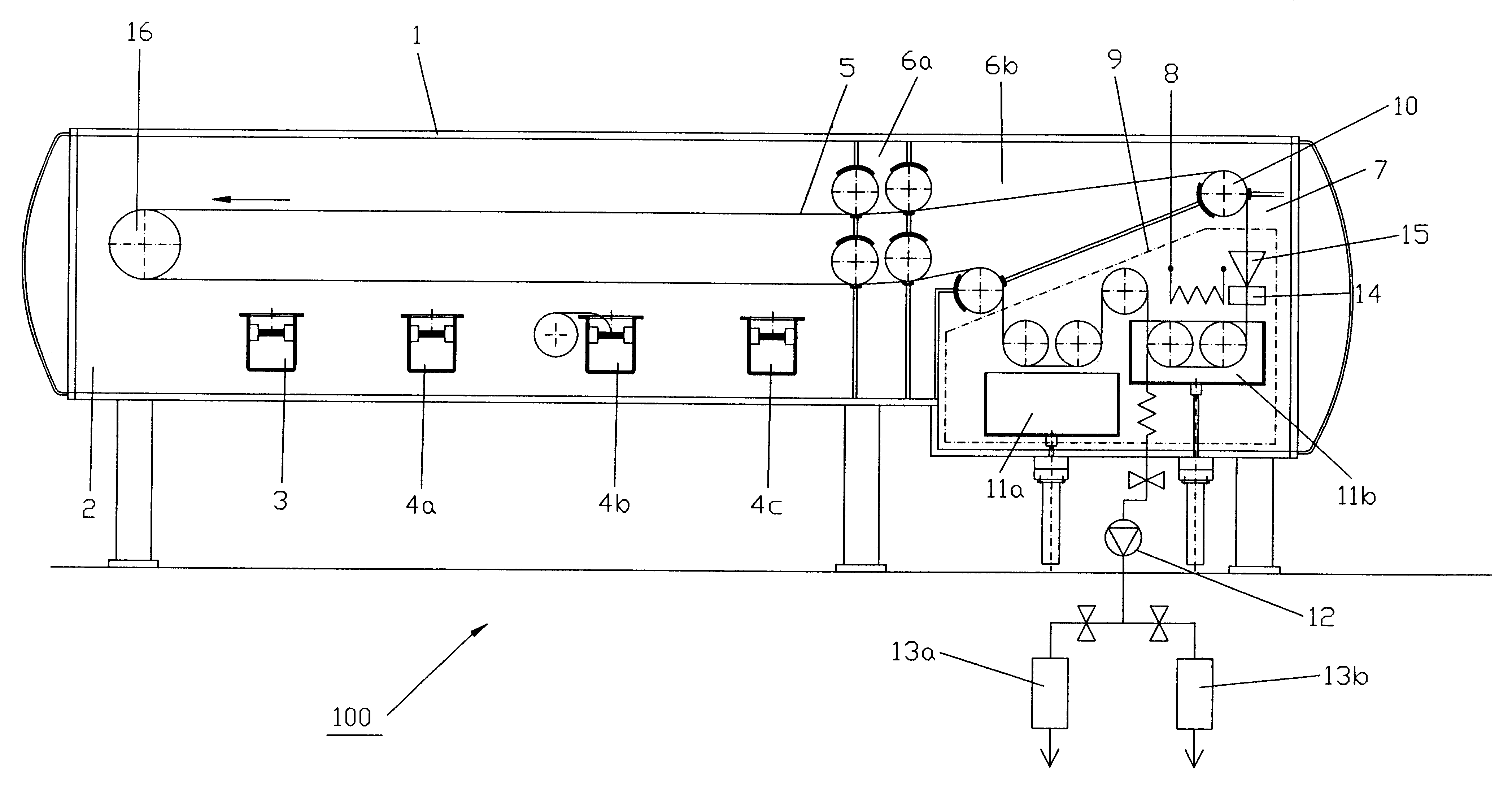
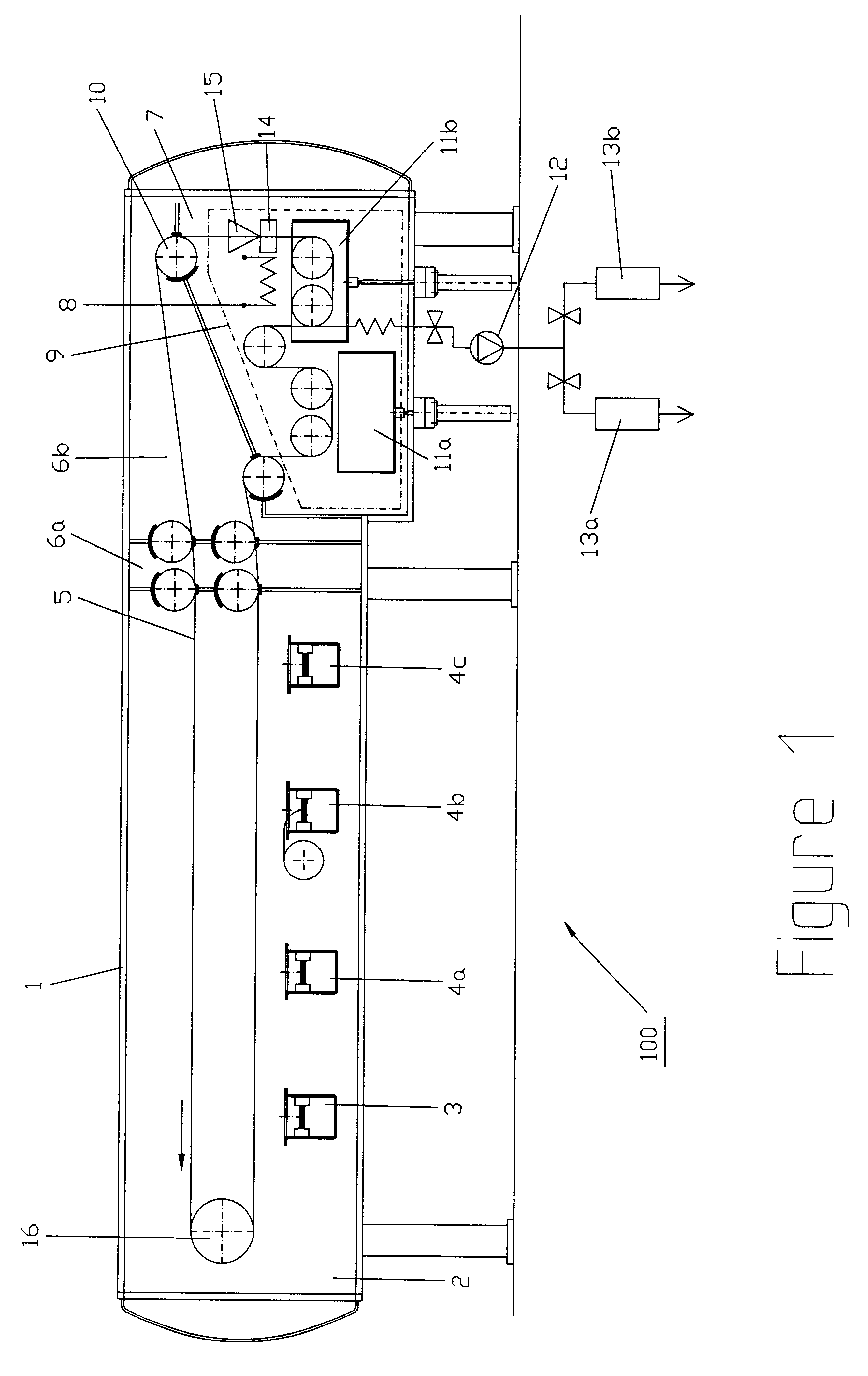
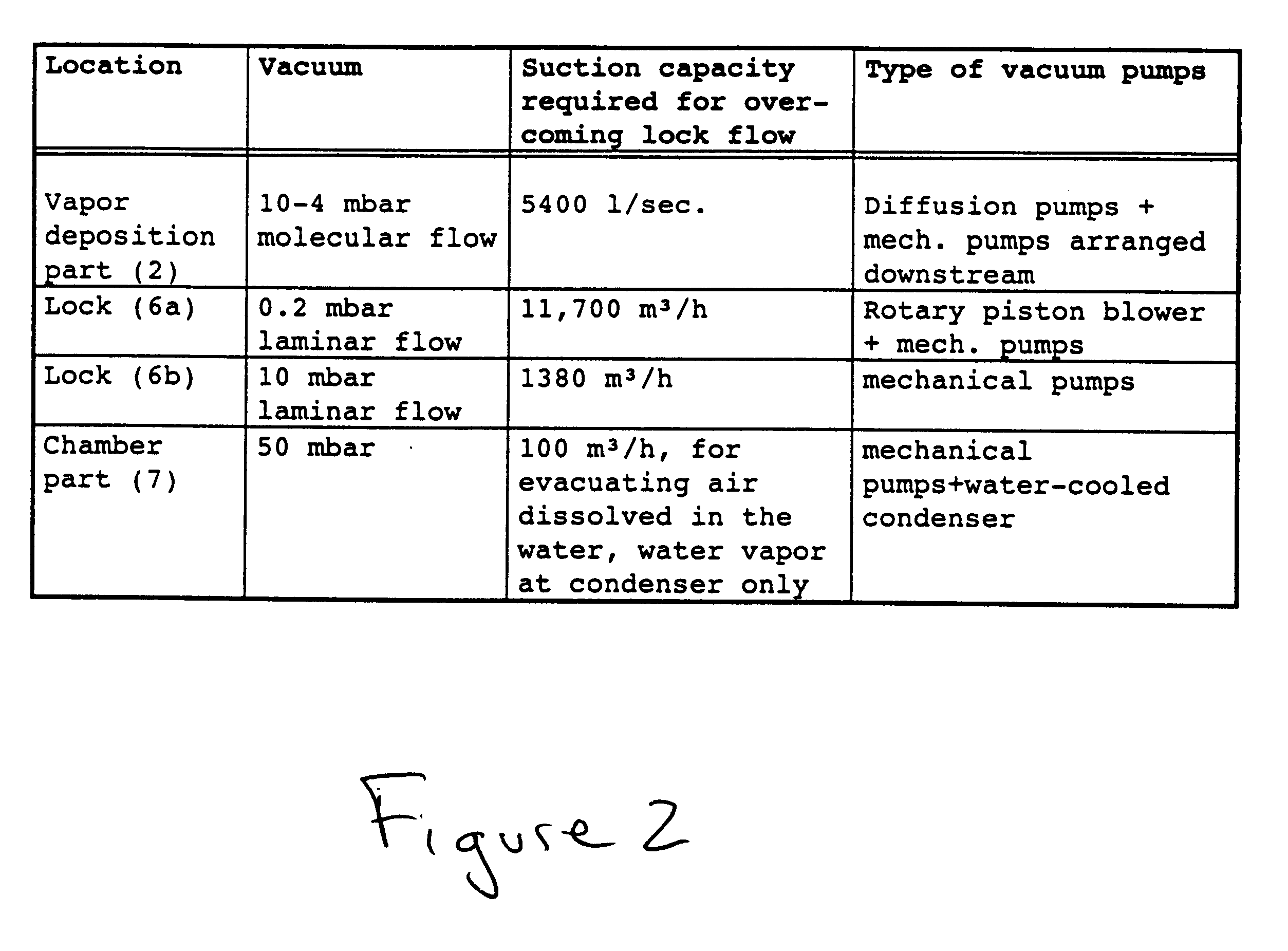
An apparatus and method technique for producing plane-parallel flakes is disclosed. In a preferred embodiment, the present invention is realized through a multi-chamber apparatus for producing plane-parallel flakes from layers vapor deposited in vacuum on an endlessly circulating substrate. The present invention includes the sequential steps of: vapor deposition of a separating agent layer in high vacuum on the endlessly circulating substrate; vapor deposition of one or more layers of metal, oxides, fluorides, and nitrides in high vacuum on the separating agent layer; and stripping the vapor deposited layers from the endlessly circulating substrate under low vacuum. The vapor deposited layers are subsequently present in a separate vacuum stage separated from the vapor deposition chamber by dynamic locks as a suspension of fine flakes in a mixture of solvent. and separating agent. The suspension may continuously or intermittently be transferred out of the separate vacuum stage for further processing. The solvent may be water in a vacuum environment of more than 20 mbar or secondary or tertiary alcohols at more than 0.05 mbar.
Owner:CIBA SPECIALTY CHEM CORP +1
Process for producing simvastatin
InactiveUS6331641B1Improve efficiencyMild conditionsOrganic chemistryBulk chemical productionHMG-CoA reductaseAlcohol
This invention provides an easy and efficient process for producing a simvastatin of great use as an HMG-CoA reductase inhibitor, which comprises deacylation of lovastatin with an inorganic base and a secondary or tertiary alcohol and subjecting the resulting diol lactone to selective protection with a ketal or acetal protective group, acylation and deprotection-lactonization to give simvastatin.
Owner:KANEKA CORP
Method for producing polybutene
InactiveUS7411104B2Easy to getHydrocarbons from unsaturated hydrocarbon additionHydrocarbonsAlcoholBoron trifluoride
A method for producing high reactive polybutene (HRPB), in which carbon-carbon double bond is positioned at an end of polybutene, is disclosed. The high reactive polybutene having 300˜5000 of number average molecular weight (Mn) can be produced from a raw material containing isobutene, wherein a polymerization reaction of the isobutene is carried out in the presence of a catalyst system including secondary alkylether, tertiary alcohol, and boron trifluoride, the amount of boron trifluoride is 0.05˜1.0 weight part per 100 weight part of isobutene, the mole ratio of a co-catalyst including secondary alkylether and tertiary alcohol:boron trifluoride is 1.0˜2.0:1, and the mole ratio of secondary alkylether:tertiary alcohol is 0.5˜1.2:1.
Owner:DL CHEM CO LTD
Process for preparing an alkoxylated alcohol or phenol
Process for preparing an alkoxylated alcohol comprising reacting a starting monohydroxy alcohol selected from secondary alcohols, tertiary alcohols and mixtures thereof with an alkylene oxide in the presence of hydrogen fluoride and a boron-containing compound comprising at least one B—O bond. The alcohol may also be a primary monohydroxy alcohol when the boron containing compound is boric acid or boric acid anhydride or a mixture thereof, or may be a primary mono hydroxy alcohol, except a C14 / C15 alcohol when reacted with ethylene oxide in the presence of HF and trimethyl borate. A phenol may be alkoxylated in the same way instead of the mono-hydroxyalcohol.
Owner:SHELL OIL CO
Method for manufacturing crystalline form I of clopidogrel hydrogen sulphate
InactiveUS20060041136A1Powder deliveryOrganic chemistryClopidogrel hydrogen sulphateHydrogen Sulfate
A method for manufacturing hydrogen sulphate (alpha S) of the alpha-(2-chlorophenyl)-6,7dihydrothieno[3,2-c]pyridine-5(4H)-acetic acid methyl ester (clopidogrel hydrogen sulphate) of formula I, in crystalline Form I, wherein the compound of formula is separated out of a solution of clopidogrel in the form of the free base or salt in a solvent selected from the series of primary, secondary or tertiary C1-C5 alcohols, their esters with C1-C4 carboxylic acids, or optionally of mixtures thereof.
Owner:ZENTIVA AS
Preparation method for 9,9-diaryl thiophene xanthene-10,10-dioxide
InactiveCN104557856AFlexible designEasy to operateOrganic chemistryNonlinear opticsOrganic synthesis
The invention discloses a preparation method for 9,9-diaryl thiophene xanthene-10,10-dioxide, and belongs to the field of fine organic synthesis and organic semiconductor material preparation. The preparation method comprises the following steps: using diphenylsulphone as a starting material and tetrahydro furan as a solvent, conducting ortho metalation reaction on diphenylsulphone under the action of n-butyllithium within a temperature range of -78-0 DEG C to obtain a corresponding aryl lithium salt; allowing the aryl lithium salt to react with an added aryl formic ether ester derivative, and performing hydrolysis to obtain a corresponding tertiary alcohol; dissolving the tertiary alcohol and electron-rich aromatic with an acetate or methylene chloride solution, using Lewis acid as a catalyst, and carrying out friedel-crafts reaction to obtain the 9,9-diaryl thiophene xanthene-10,10-dioxide. The preparation method has the advantages that the reactions are easy to control, the operation is simple, the cost is low, the repeatability is good, the productivity is high, and the product quality is high; an organic modular unit and an organic functional semiconductor can be conveniently made; the 9,9-diaryl thiophene xanthene-10,10-dioxide is used in the fields of organic light emitting diodes, organic transistors, organic laser, and organic nonlinear optics, and the like.
Owner:NANJING UNIV OF POSTS & TELECOMM
Acrylate-containing polymer blends and methods of using
InactiveUSRE37036E1Improve adhesionGroup 4/14 element organic compoundsLiquid surface applicatorsMethacrylatePolymer science
A polymer blend comprising (a) a modified block copolymer comprising (i) a polystyrene block and (ii) a polydiene block or a hydrogenated polydiene block, said polydiene block or hydrogenated polydiene block being modified to contain an average of one or more carboxyl groups; and (b) a polymer comprising a polymerization reaction product of two or more mono-ethylenically unsaturated monomers in which (i) at least one of the monomers is an acrylic or methacrylic acid ester of a non-tertiary alcohol having 1 to 14 carbon atoms, inclusive and (ii) at least one of the monomers has carboxylic acid functionality and is present in an amount ranging from about 1 to about 15 parts by weight, based on 100 parts by weight of polymer (b), and a method of priming a substrate comprising applying the blend to the substrate.
Owner:3M INNOVATIVE PROPERTIES CO
Method for preparing low-unsaturation-degree polyether polylol
The invention relates to the preparation of a kind of low unsaturation polyether polyols. It's mainly used to solve the problem in previous technology that it has long catalyst inductive period and the concentration of catalyst in initiator is high, which is that it has a high catalyst cost in the preparation of unsaturation polyether polyols. The invention excellently solves the said problem through adopting catalyst components containing double metal cyanide (DMC) mixture, C4 - C10 organic mellow with tertiary alcohol structure, adding bronsted acid, and adopting the technology project of inert gas frothing to degasificate. So it can be used in the industrial production of low unsaturation polyether polyols.
Owner:SINOPEC SHANGHAI GAOQIAO PETROCHEM CORP +1
Modified Retinoid Compounds and Their Uses
InactiveUS20080194534A1Low toxicityGreat therapeutic windowBiocideCosmetic preparationsDiseaseRetinoid
A method of minimizing or reducing the toxicity of a retinoid having a free carboxyl group and the resulting modified retinoids are described. The method comprises the step of esterifying the carboxyl group of the retinoid with a highly sterically hindered compound, which is preferably a secondary or tertiary alcohol. The resulting retinoid esters are rendered much less toxic than the starting or parent retinoid. This process provides a retinoid ester analog of reduced toxicity so that it may be administered orally with minimal side effects and with a much greater therapeutic window. The modified retinoid compounds are useful in the treatment and prophylaxis of all diseases and disorders where retinoid compounds have been shown effective.
Owner:WISCONSIN ALUMNI RES FOUND
Process for the production of polyols using DMC catalysts having unsaturated tertiary alcohols as ligands
The present invention provides an active double metal cyanide (DMC) catalyst made of a non-hexanitrometallate containing double metal cyanide compound, one or more unsaturated tertiary alcohols and about 0 to about 80 wt. %, based on the amount of catalyst, of a functionalized polymer having a number average molecular weight greater than about 200. Also provided are methods of producing the inventive catalysts. The inventive catalysts may find use in the production of polyols.
Owner:COMBS GEORGE
Process for producing polyether polyols with low degree of unsaturation
The invention relates to a process for producing polyether polyols with low degree of unsaturation, wherein the catalyst composition comprises bimetallic cyanide mixture, C4-C10 organic alcohol having a tertiary alcohol structure and selected from silicon acid esters or aliphatic esters, aromatic diester and organic esters of their mixtures, the catalyst can be applied into the industrial production of polyether polyols.
Owner:CHINA PETROLEUM & CHEM CORP +1
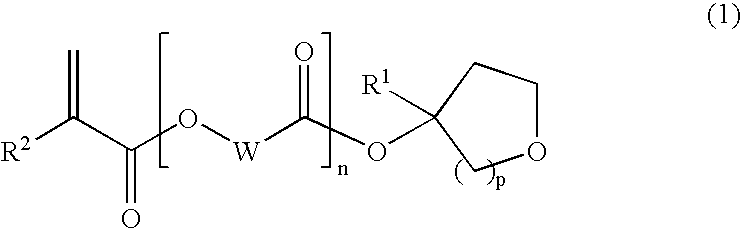
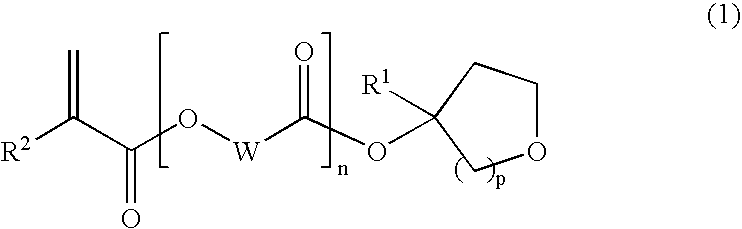
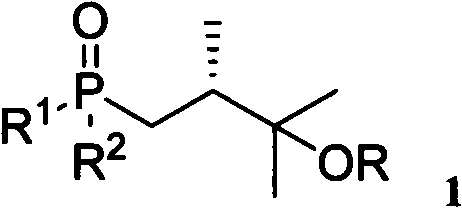
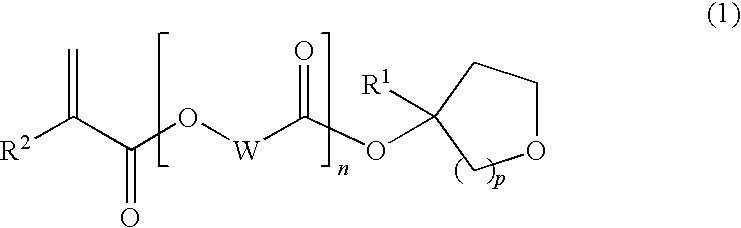
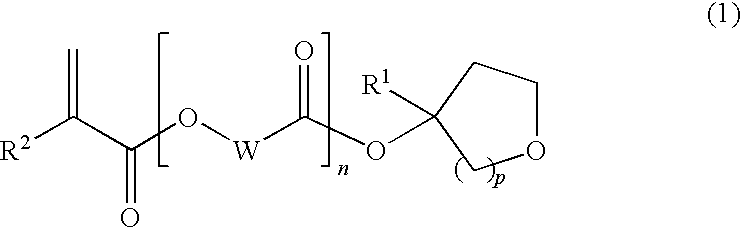
Tertiary alcohol derivative, polymer compound and photoresist composition
InactiveUS20090035700A1High dissolution rateSmall swellingOrganic chemistryPhotosensitive materialsResistHydrogen atom
(1) A polymer compound for photoresist compositions which is high in storage stability and small swelling at the development and (2) a compound which is a raw material for such a polymer compound are provided; and (3) a photoresist composition with improved LWR containing the subject polymer compound are further provided. In detail, [1] a tertiary alcohol derivative represented by the following general formula (1) is provided.(In the formula, wherein R1 represents a linear alkyl group having from 1 to 6 carbon atoms, a branched alkyl group having from 3 to 6 carbon atoms or a cyclic alkyl group having from 3 to 6 carbon atoms; R2 represents a hydrogen atom or a methyl group; W represents a linear alkylene group having from 1 to 10 carbon atoms, a branched alkylene group having from 3 to 10 carbon atoms or a cyclic alkylene group having from 3 to 10 carbon atoms; n represents 0 or 1; and p represents 1 or 2.)
Owner:KURARAY CO LTD
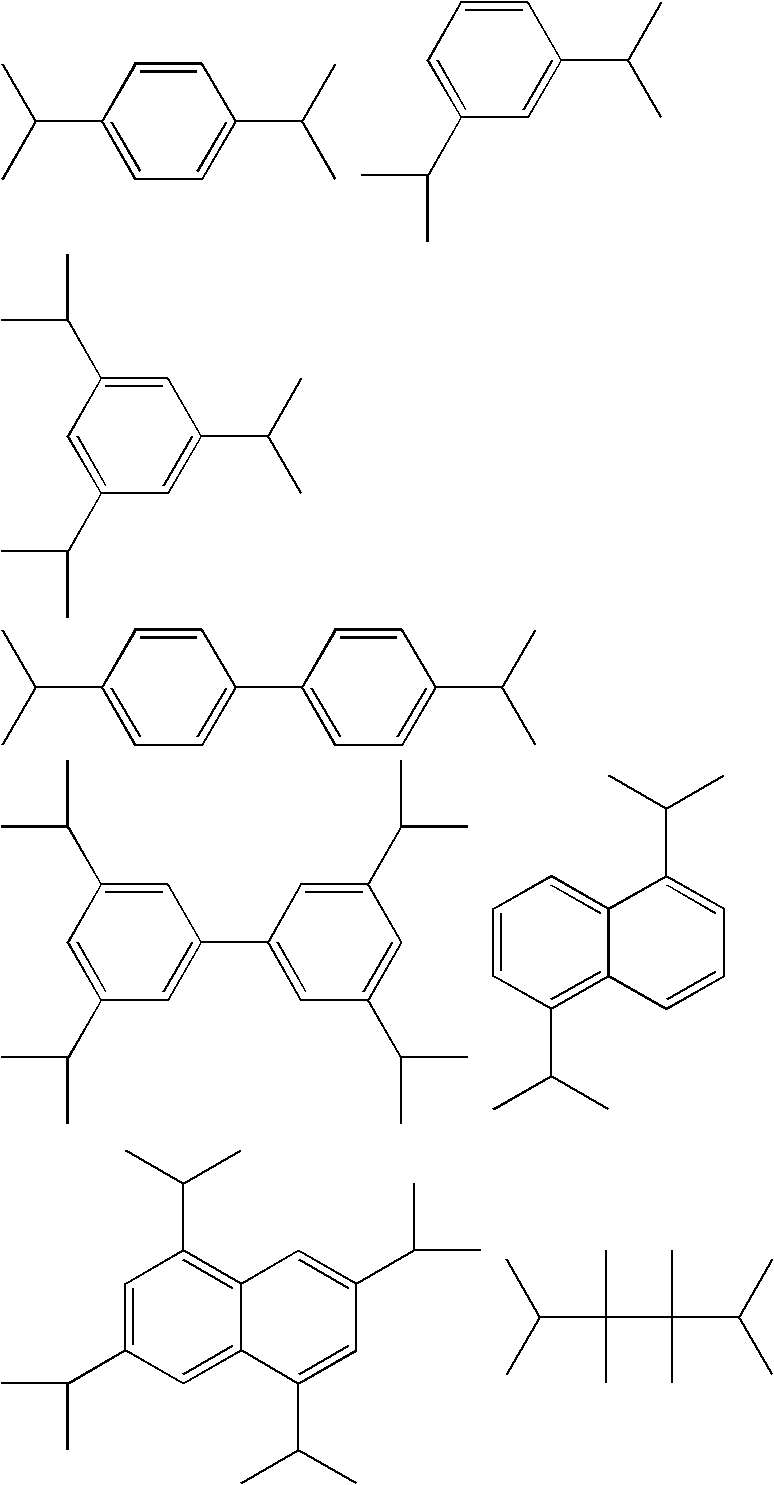
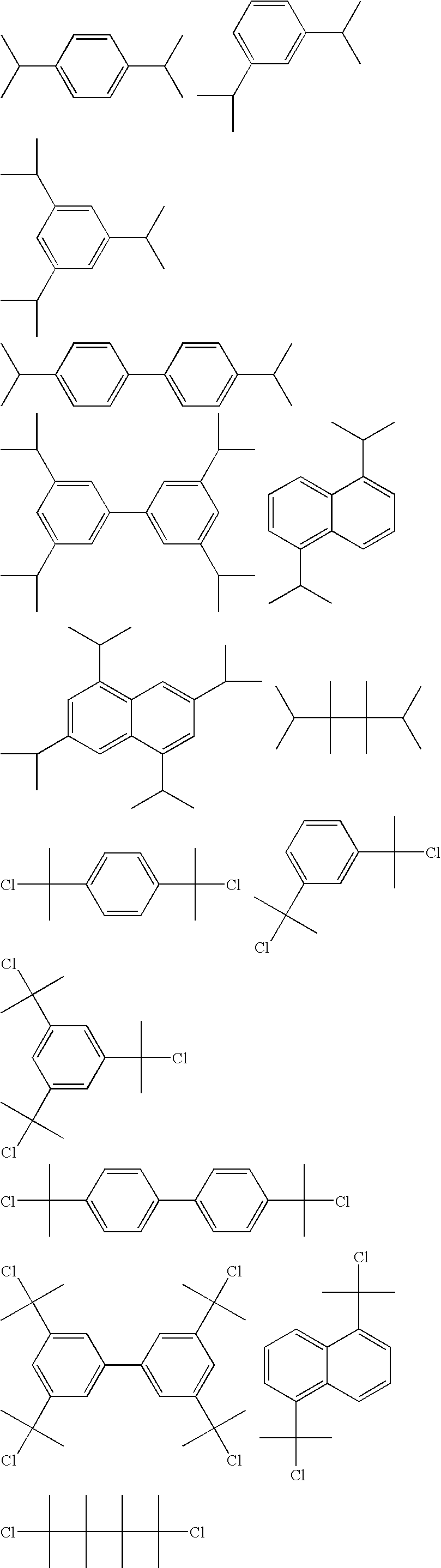
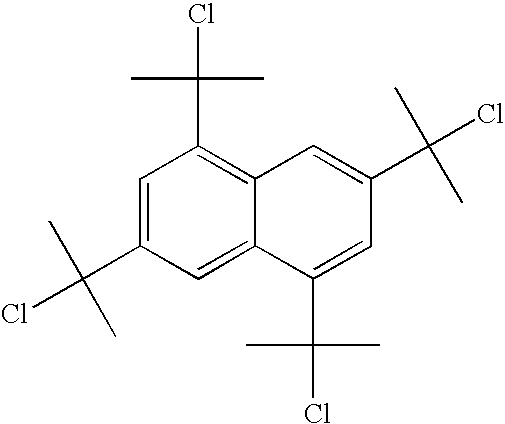
Process for chlorinating tertiary alcohols
InactiveUS20050119349A1Possible to separateReduce in quantityOrganic active ingredientsBiocideSimple Organic CompoundsO-Xylene
Owner:GRUNENTHAL GMBH
Preparation of biuret-containing polyisocyanates
InactiveUS7022874B2Easy to disassembleIncrease resistanceUrea derivatives preparationIsocyanic acid derivatives preparationArylAlcohol
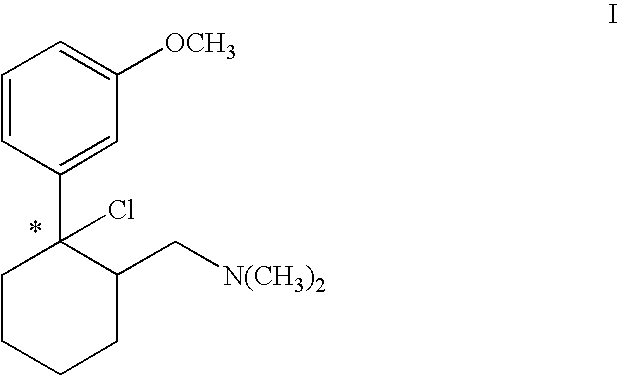
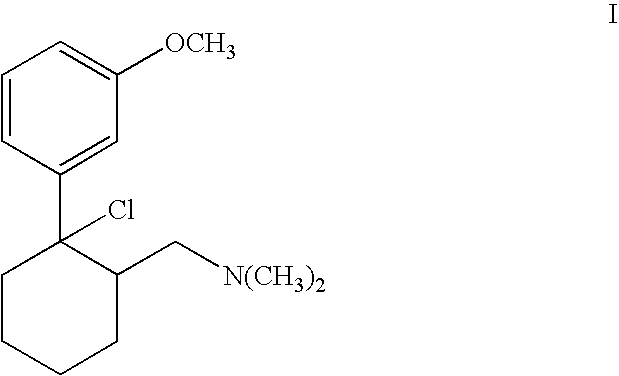
A process for the preparation of polyisocyanates which contain one or more biuret groups, by reactinga) an aliphatic or cycloaliphatic isocyanate containing two or more isocyanate groups (isocyanate a) withb) a tertiary alcohol or a mixture of water and a tertiary alcohol (biuretizing agent b)at from 100 to 250° C., which comprises carrying out the reaction in the presencec) of a stabilizer (c) which constitutes a catalytic amount of urea, ammonia, biuret, a urea derivative of the formula I in which R1, R2, R3 and R4 are hydrogen, C1 to C10 alkyl or C5 to C10 aryl, ora carboxamide of the formula II in which R5 is C1 to C12 alkyl which is unsubstituted or in which 1, 2 or 3 hydrogen atoms are replaced by a radical
Owner:BASF AG
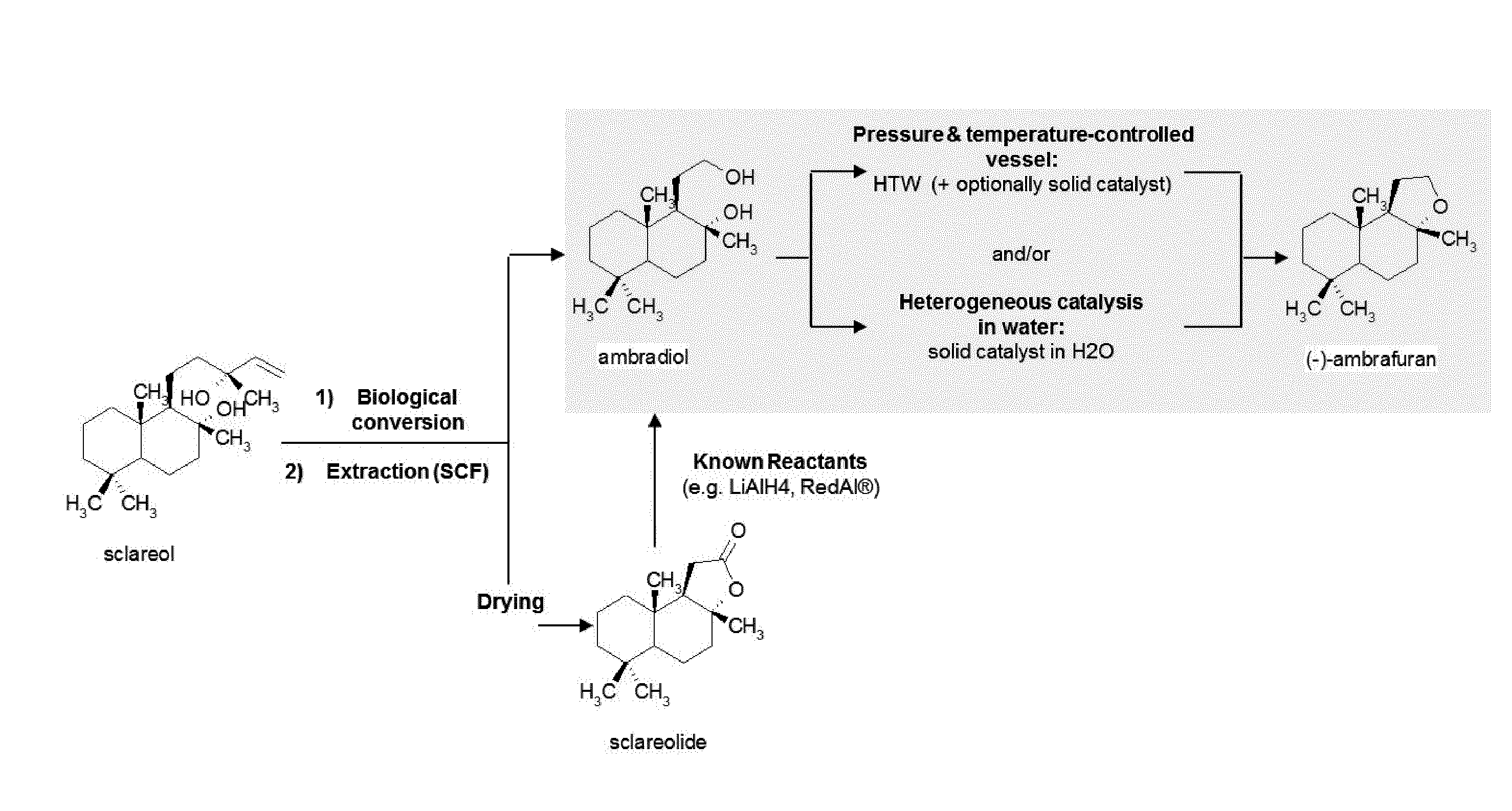
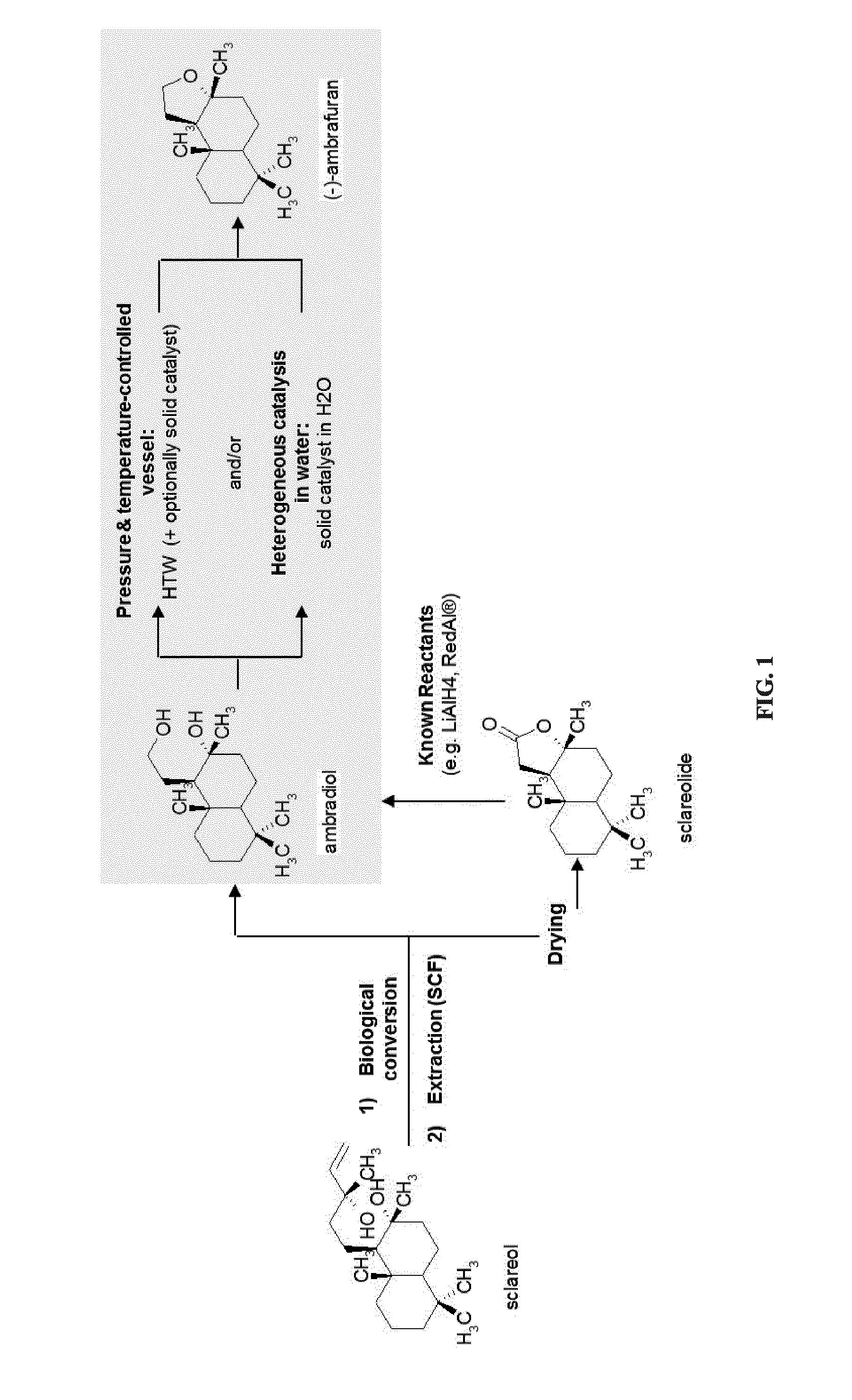
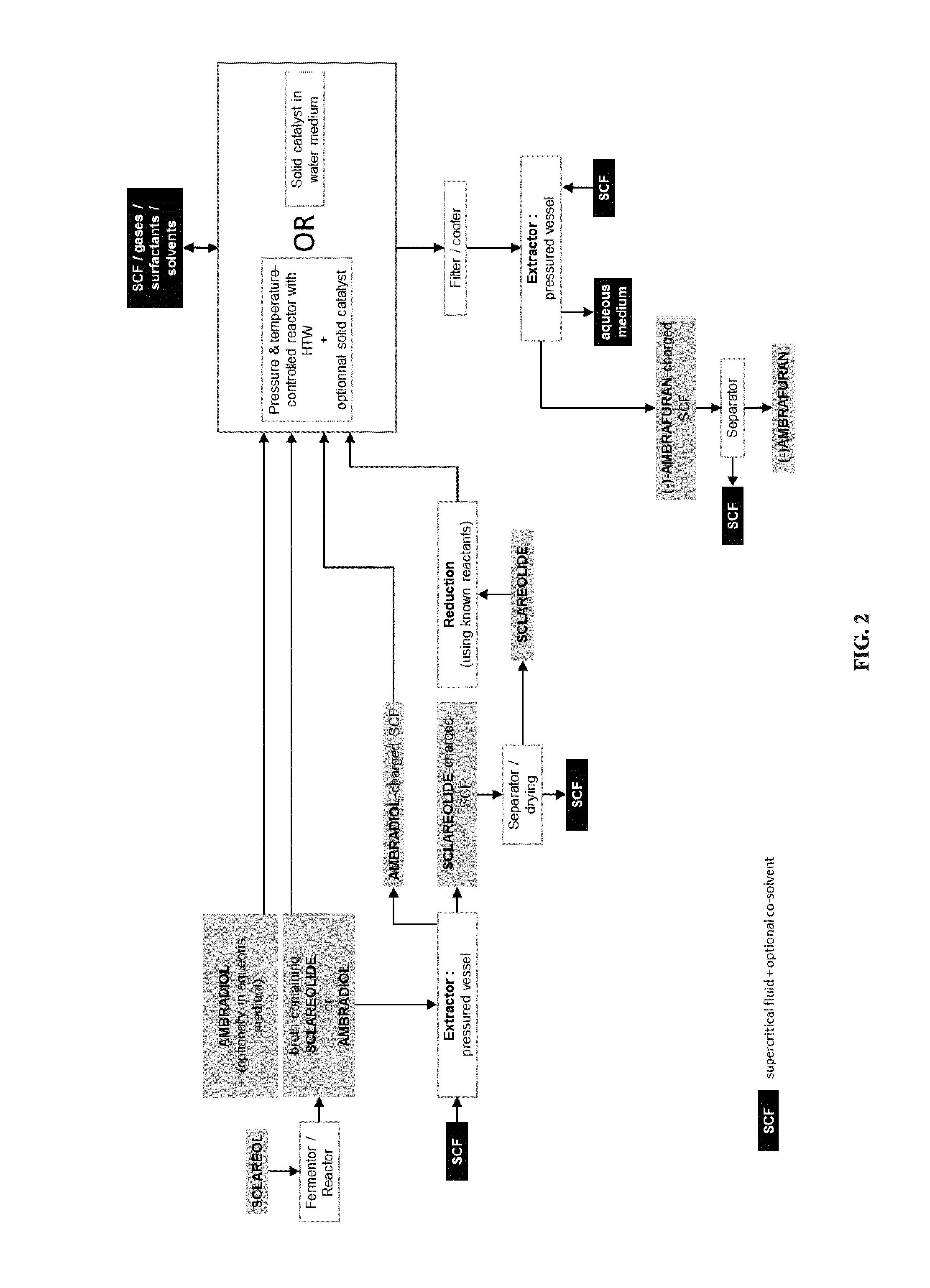
Process for the cyclodehydration of diols and use thereof for the manufacturing of ambrafuran and other cycloether derivatives
ActiveUS20140199741A1Pressurized chemical processOrganic compound preparationTetrahydropyranChemistry
A process for manufacturing tetrahydrofuran, tetrahydropyran and, more generally, cycloether derivatives through the cyclodehydration of 1,4- or 1,5-diols. More specifically, the process of the invention involves (i) the stereoselective cyclodehydration in water of 1,4- or 1,5-diols including at least one chiral tertiary alcohol functional group with retention of the initial chirality, and / or (ii) the cyclodehydration in water of 1,4- or 1,5-diols, said diols being non-miscible with and / or non-soluble in water, into corresponding cycloether derivatives, by bringing the reaction mixture to high temperature water (HTW) conditions and / or by mixing the aqueous reaction mixture with a solid catalyst, such as for example a smectite clay. Also, the use of the process for manufacturing ambrafuran, especially (−)-ambrafuran and other cycloether derivatives.
Owner:KOSTE BIOCHEM
Method for synthesizing (E,E) Geranyl linalool
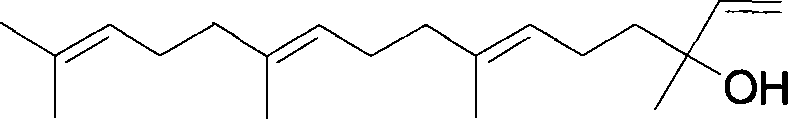
InactiveCN101070270AOrganic compound preparationHydroxy compound preparationSulfonyl chlorideNerolidol
This invention relates to synthetic method of a ( E, E) - geranyl linalool. The invention takes (E) - nerolidol as raw material. The hydroxyl is shield by dihydropyrane, gain ( E) - nerolidol tetrahydropyrane aether; selenium dioxide and teri-butyl hydroperoxide selectively oxidize the anti-form methyl of ( E) - nerolidol tetrahydropyrane aether to gain anti-form allyl position hydroxylated oxidative product ( E, E) - 12 - hydroxy nerolidol tetrahydropyrane aether, transit halogenating reaction to gain ( E, E) - 12 - halogeno- nerolidol tetrahydropyrane aether, then take reaction with isopropyl methyl ketone that is selectively divested one proton by diisopropyl amido lithium, generate ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - ketone, use sodium borohydride to reduce to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - alcohol, takes reaction with sulfonyl chloride or sulphonic acid ester with alkali presence to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 oxygen) -16 - 6, 10, 15 - triene - 3 - alcoholic sulphonic acid ester, then divide sulphonic acid ester group under base catalysis to gain ( E, E) - geranyl linalool tetrahydropyrane aether, and by deprotection to gain ( E, E) - geranyl linalool. ËFor the configuration of ( E) - nerolidol 3 position tertiary carbon is not influenced in the course of reaction, if use ( E) - nerolidol that has optical activity as raw material, should gain optical active ( E, E) - geranyl linalool. ((E, E)-geranyl linalool can replace Teprenone and such type medicament intermediate, natural product intermediate, insect pheromone and spice etc.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
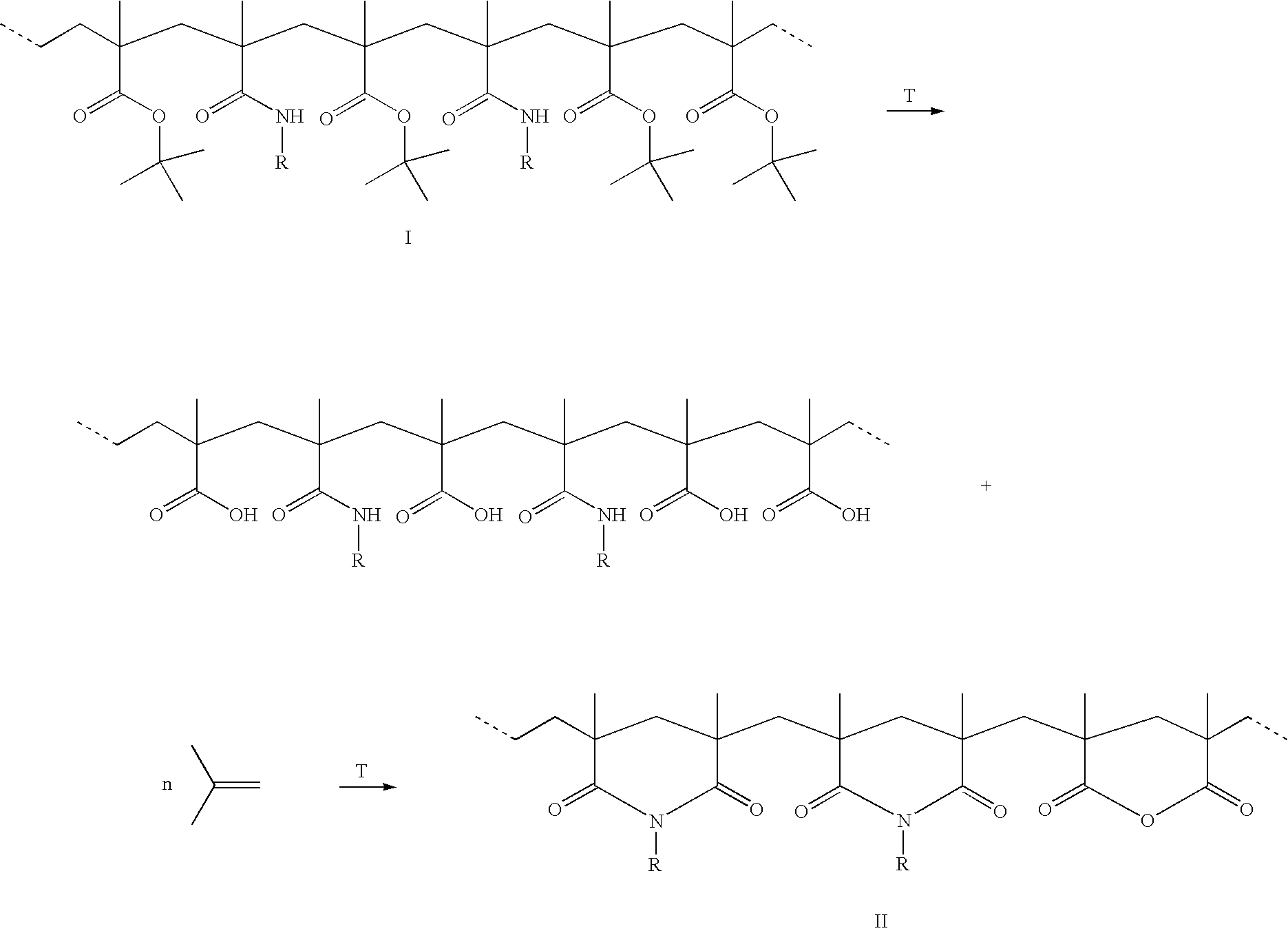
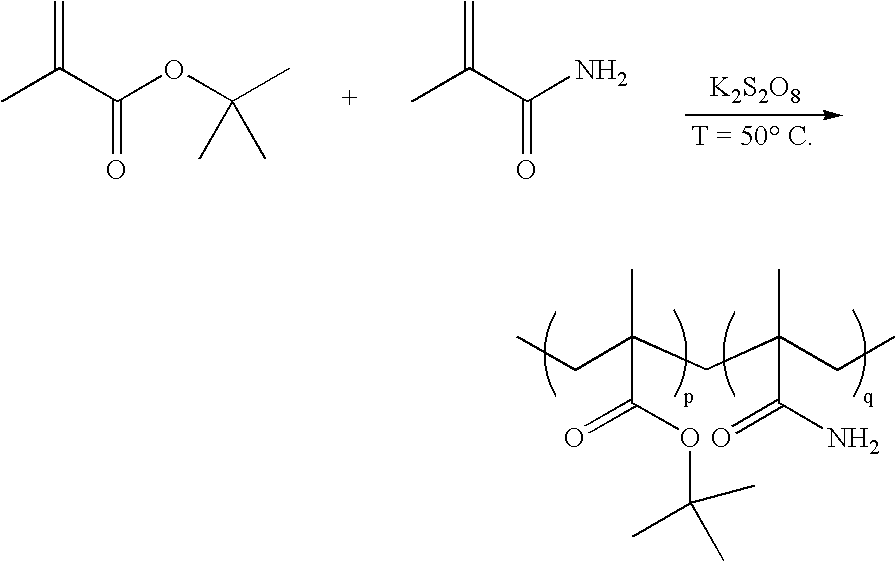
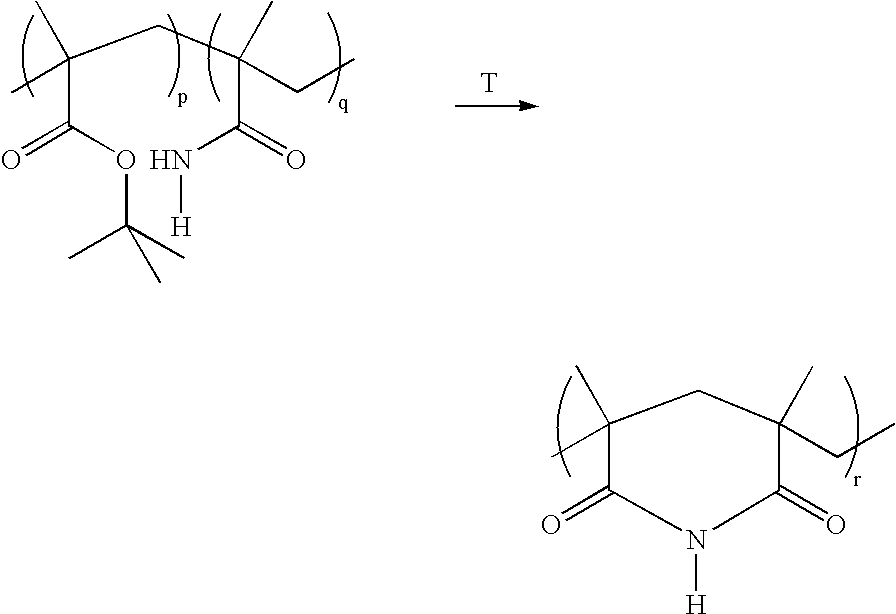
Method for the synthesis of copolymers for producing polymethacrylimides
The invention relates to a method for producing polymethacrylimides in two steps: 1) radical copolymerization of (meth)acrylamides (A, (Me,H)HC=CHCONHR2) and alkyl(meth)acrylic esters (B) and optionally further ethylenically unsaturated monomers in the presence of an aqueous solvent. The monomers (A) include, in addition to acrylamide and methacrylamide, (meth)acrylamides that are substituted on their nitrogen group (R2<>H). The monomers (B) are the (meth)acrylic esters of secondary or tertiary alcohols, preferably tert. butylmethacrylate. 2) Thermal or catalytic reaction of the copolymers produced in 1) to polymethacrylimide or for R2<>H to N-substituted polymethacrylimides while alkenes are separated.
Owner:ROHM GMBH
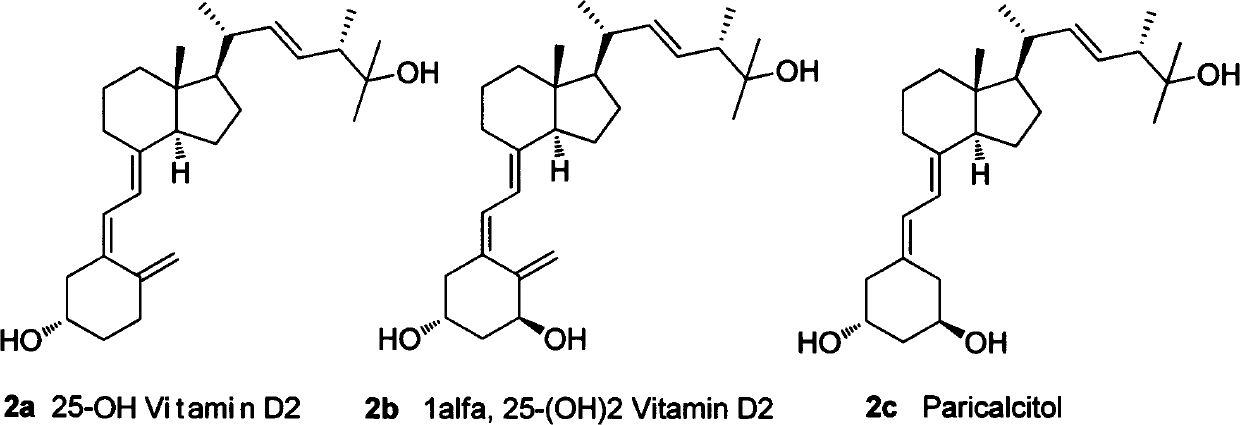
25-hydroxy vitamin D2 series medicament side chain and its preparation method
InactiveCN102351901AWide range of usesEasy to storeGroup 5/15 element organic compounds3-Hydroxypropionic acidSide chain
The invention relates to a 25-hydroxy vitamin D2 series medicament side chain and its preparation method. The invention provides a novel compound 1, the structure of which is shown in formula 1, and a synthetic method thereof. The synthetic method provided by the invention comprises the following steps of: using (S)-2-methyl-3-hydroxypropionic acid methyl ester as an initial raw material, performing a reaction between (S)-2-methyl-3-hydroxypropionic acid methyl ester and a methyl metal reagent, carrying out selective halogenation or sulfonate esterification, followed by selective halogenation and protection of tertiary alcohols, and performing a reaction with a metallic compound of diaryl phosphine or trialkoxyl phosphine to obtain the target compound.
Owner:SHANGHAI HAOYUAN CHEMEXPRESS
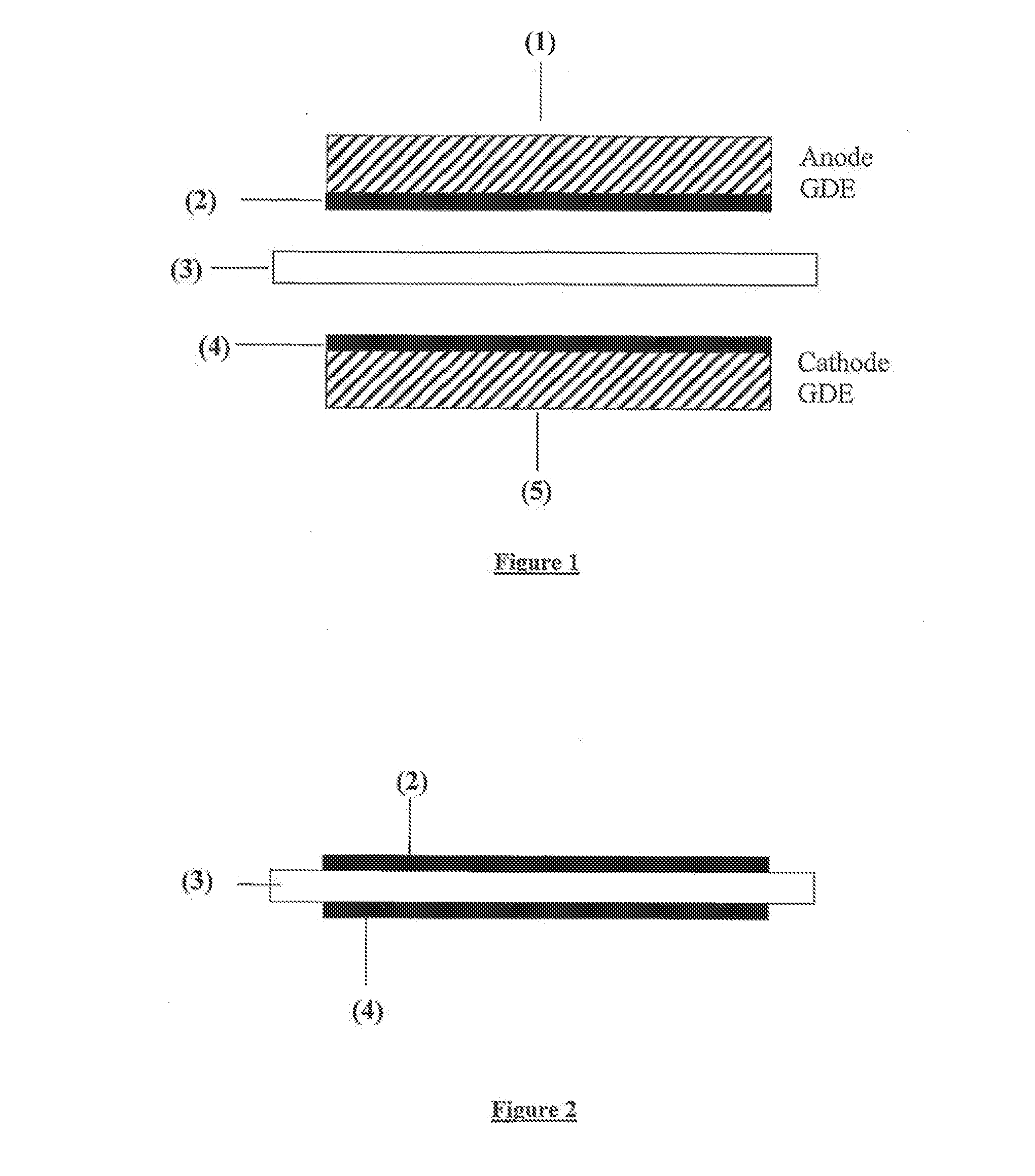
Ink for producing catalyst layers
ActiveUS8198206B2Stable to oxidative degradationInhibition formationCellsCatalyst protectionIonomerOrganic solvent
The invention relates to an ink for producing catalyst layers for electrochemical devices. The ink comprises catalyst materials, ionomer material, water and at least one organic solvent. The organic solvent belongs to the class of tertiary alcohols and / or the class of aliphatic diketones and bears functional groups which are stable to oxidative degradation in the ink. This prevents formation of decomposition products in the ink. The ink of the invention displays a high storage stability and is used for producing catalyst-coated substrates for electrochemical devices, in particular fuel cells (PEMFCs, DMFCs).
Owner:UMICORE AG & CO KG
Preparation method and application of chiral tertiary alcohol or tertiary ether compound
ActiveCN107382644AHigh regional selectivityHigh stereoselectivityCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystEnantio selectivity
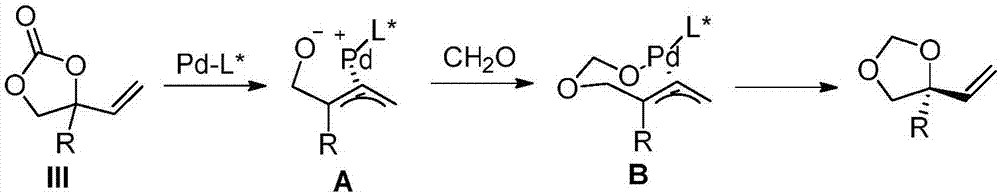
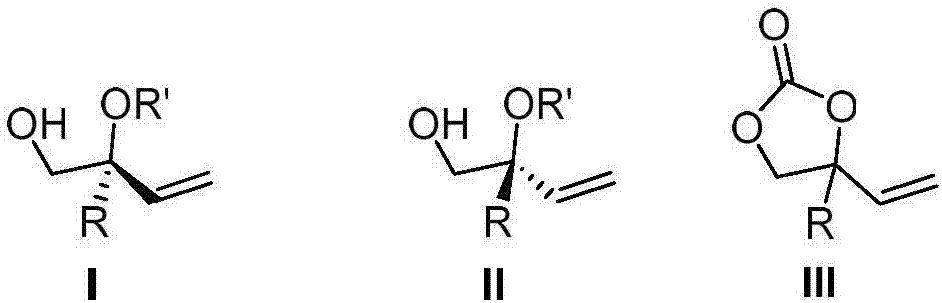
The invention discloses a preparation method and application of a chiral tertiary alcohol or tertiary ether compound. A racemase 4-substituted-4-vinyl-1,3-dioxolan-2-one compound is used as a raw material, and under the catalysis of a palladium coordination compound, which is generated under a palladium source and the coordination action of a chiral ligand, and a boron compound which serve as catalysts, reacts with water or alcohol to prepare the chiral tertiary alcohol or tertiary ether compound. The chiral compound provided by the invention is the multi-functionalized chiral tertiary alcohol or tertiary ether compound, can be used for flexibly and conveniently carrying out functional conversion, is about to be an important chiral molecular building block for preparing a chiral drug and an intermediate. The preparation method provided by the invention comprises asymmetric hydroxylation and etherification reactions co-catalyzed by palladium and boron; the preparation method is high in catalytic activity, high in regioselectivity and enantioselectivity and mild in reaction condition; reactive raw materials are conveniently and easily obtained.
Owner:SHANGHAI JIAO TONG UNIV
Method for synthesizing alpha - olefinic bond functional cyclic carbonate from alpha ¿C alkynyl tertiary carbon alcohol and carbon dioxide
This invention relates to a synthesis method for alpha-olefinic bond-functionalized cyclic carbonates from alpha-alkynyl tertiary alcohols and carbon dioxide. In this method, ionic liquids which consist of alkylpyridines or 1,3-dialkylimidazole cations and inorganic or organic anions and have a liquid state at room temperature are adopted as reaction media, univalent or bivalent copper salts are adopted as catalysts; and alpha-alkynyl tertiary alcohols and carbon dioxide are catalyzed to react with each other at a pressure of 0.1~2.0MPa and a temperature of 40~180 deg.C for 1~20 hours so as to produce alpha-olefinic bond-functionalized cyclic carbonates. It is characteristics of this method that no more organic solvents or organic bases are needed, and compared to conventional catalytic process; it has the advantages of low carbon dioxide pressure, high reaction rate, high product purity and reusability of ionic liquids and is thus pretty promising for industrial application.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Multi-metal cyanide complex catalyst
The invention relates to a polymetallic cyanide complex catalyst for ring opening polymerization of oxyalkylene, mainly aiming at resolving the problems existing in the prior technique that the activity of a catalyst is not enough and the catalyst needs relatively long induction period to acquire higher activity. In the invention, the technical proposal which better resolves the problems is as follows: the catalyst comprises one or a plurality of metallic cyanide complexes, a C4 to C10 organic alcohol with an tertiary alcohol structure and an organic ligand selected from aliphatic ester, aromatic monoester or aromatic diester and a mixture of the aromatic monoester and the aromatic diester. The catalyst has the advantages of no induction period and high activity, and is applied to the industrial preparation of unsaturated polyether polyols by the ring opening polymerization of the oxyalkylene.
Owner:CHINA PETROLEUM & CHEM CORP +1
Double metal cyanide catalysts
InactiveCN1500554AShort induction periodHigh catalytic activityOrganic-compounds/hydrides/coordination-complexes catalystsCyanide compoundAlcohol
The present invention relates to one kind of double-metal cyanide catalyst and aims at solving the problem of catalyst with longer induction period. The catalyst includes double-metal cyanide mixture, organic C4-C10 alcohol with tertiary alcohol structure and organic ester selected from fatty ester, aromatic monoester, aromatic biester and their mixture. It may be used in the industrial production of low unsaturation degree polyether polyol.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for producing chlorinated hydrocarbon having chlorinated tertiary carbon
InactiveUS20050250968A1Easy to produceOrganic halogenationHalogenated hydrocarbon separation/purificationHypochloriteCationic polymerization
The present invention relates to a simple method for efficiently producing aromatic-substituted chlorinated hydrocarbons, for example, high-purity cumyl chloride (1,4-bis(2-chloro-2-propyl)benzene, p-DCC) that can be used as an initiator for cationic polymerization. A corresponding tertiary alcohol such as 1,4-bis(2-hydroxy-2-propyl)benzene is mixed with aqueous hydrochloric acid and subjected to stirring, and then the resulting organic layer is brought into contact with a hydrogen chloride gas to produce high-quality aromatic-substituted chlorinated hydrocarbon in high yield. Furthermore, in order to purify a mixture containing a chlorinated hydrocarbon compound, the mixture being produced by reaction between an aqueous solution of a metal hypochlorite and a protonic acid, the mixture is allowed to react with an aqueous alkaline solution to form an alcohol compound. Then, a solid is isolated by solid-liquid separation and chlorinated again with the aqueous hydrochloric acid. As a result, a high-purity chlorinated hydrocarbon compound is produced in high yield.
Owner:KANEKA CORP
Ink For Producing Catalyst Layers
ActiveUS20110166009A1Stable to oxidative degradationInhibition formationCellsCatalyst protectionIonomerDiketone
The invention relates to an ink for producing catalyst layers for electrochemical devices. The ink comprises catalyst materials, ionomer material, water and at least one organic solvent. The organic solvent belongs to the class of tertiary alcohols and / or the class of aliphatic diketones and bears functional groups which are stable to oxidative degradation in the ink. This prevents formation of decomposition products in the ink. The ink of the invention displays a high storage stability and is used for producing catalyst-coated substrates for electrochemical devices, in particular fuel cells (PEMFCs, DMFCs).
Owner:UMICORE AG & CO KG
Tertiary alcohol derivative, polymer compound and photoresist composition
(1) A polymer compound for photoresist compositions which is high in storage stability and small swelling at the development and (2) a compound which is a raw material for such a polymer compound are provided; and (3) a photoresist composition with improved LWR containing the subject polymer compound are further provided. In detail, [1] a tertiary alcohol derivative represented by the following general formula (1) is provided.(In the formula, wherein R1 represents a linear alkyl group having from 1 to 6 carbon atoms, a branched alkyl group having from 3 to 6 carbon atoms or a cyclic alkyl group having from 3 to 6 carbon atoms; R2 represents a hydrogen atom or a methyl group; W represents a linear alkylene group having from 1 to 10 carbon atoms, a branched alkylene group having from 3 to 10 carbon atoms or a cyclic alkylene group having from 3 to 10 carbon atoms; n represents 0 or 1; and p represents 1 or 2.)
Owner:KURARAY CO LTD
Method for preparing N-long chain acyl neutral amino acid
InactiveUS20030125227A1Efficient conductionLow viscosityCosmetic preparationsHair cosmeticsNeutral Amino AcidsAlanine
A method for preparing a highly purified N-long chain acyl neutral amino acid for use of a detergent and the like in a simple and convenient manner and in a high yield by reacting a neutral amino acid such as glycine, gamma-aminobutyric acid, and alanine, with a saturated or unsaturated fatty acid halide having 8 to 22 carbon atoms, wherein the reaction is performed in a mixture of water and one or more kinds of hydrophilic organic solvents selected from the group consisting of acetone, acetonitrile, a secondary alcohol having 3 or 4 carbon atoms, and a tertiary alcohol having 4 carbon atoms such as isopropanol, sec-butanol, and tert-butanol in the presence of a base.
Owner:AJINOMOTO CO INC
Process for producing fluorinated polyvalent carbonyl compound
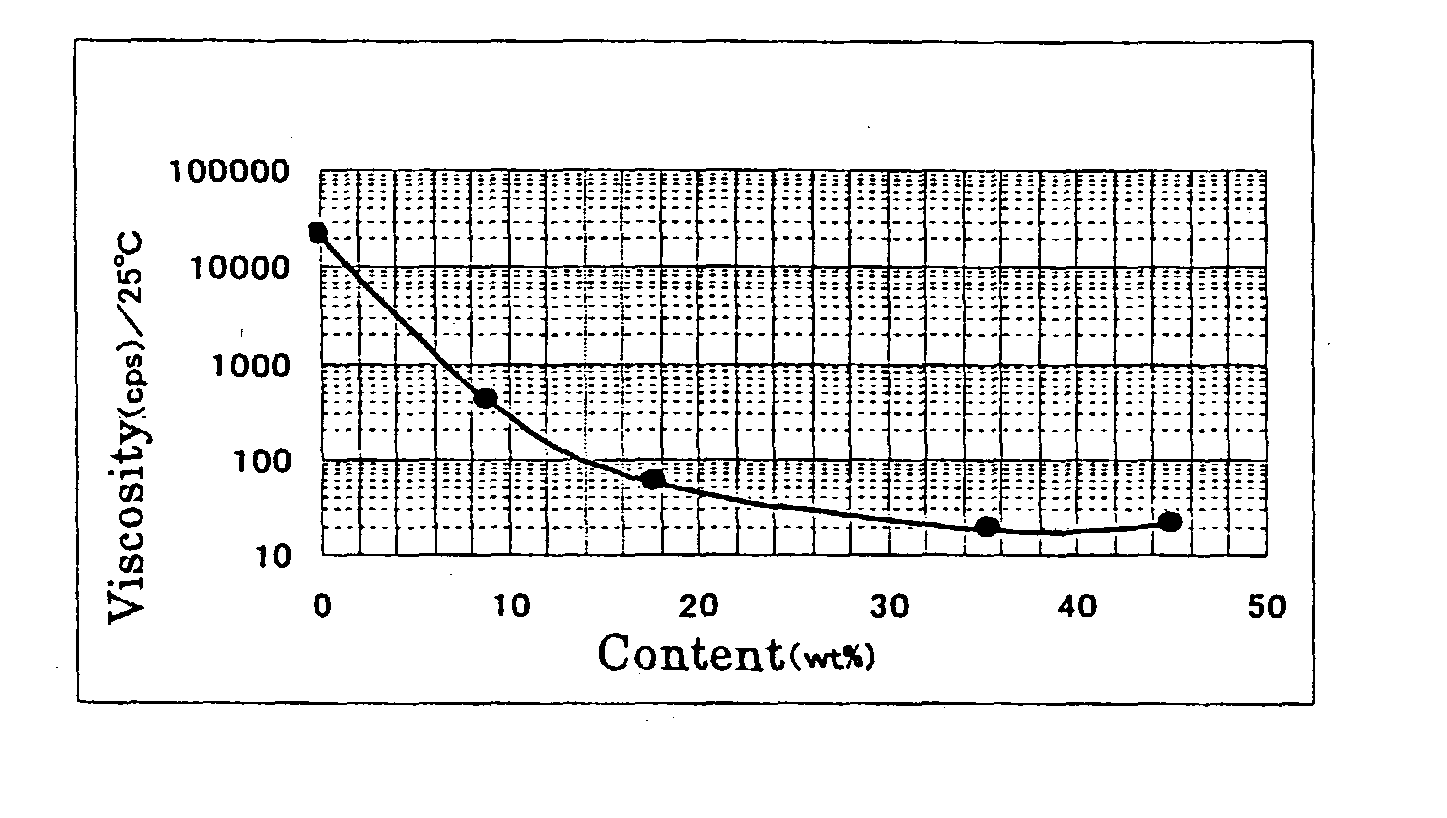
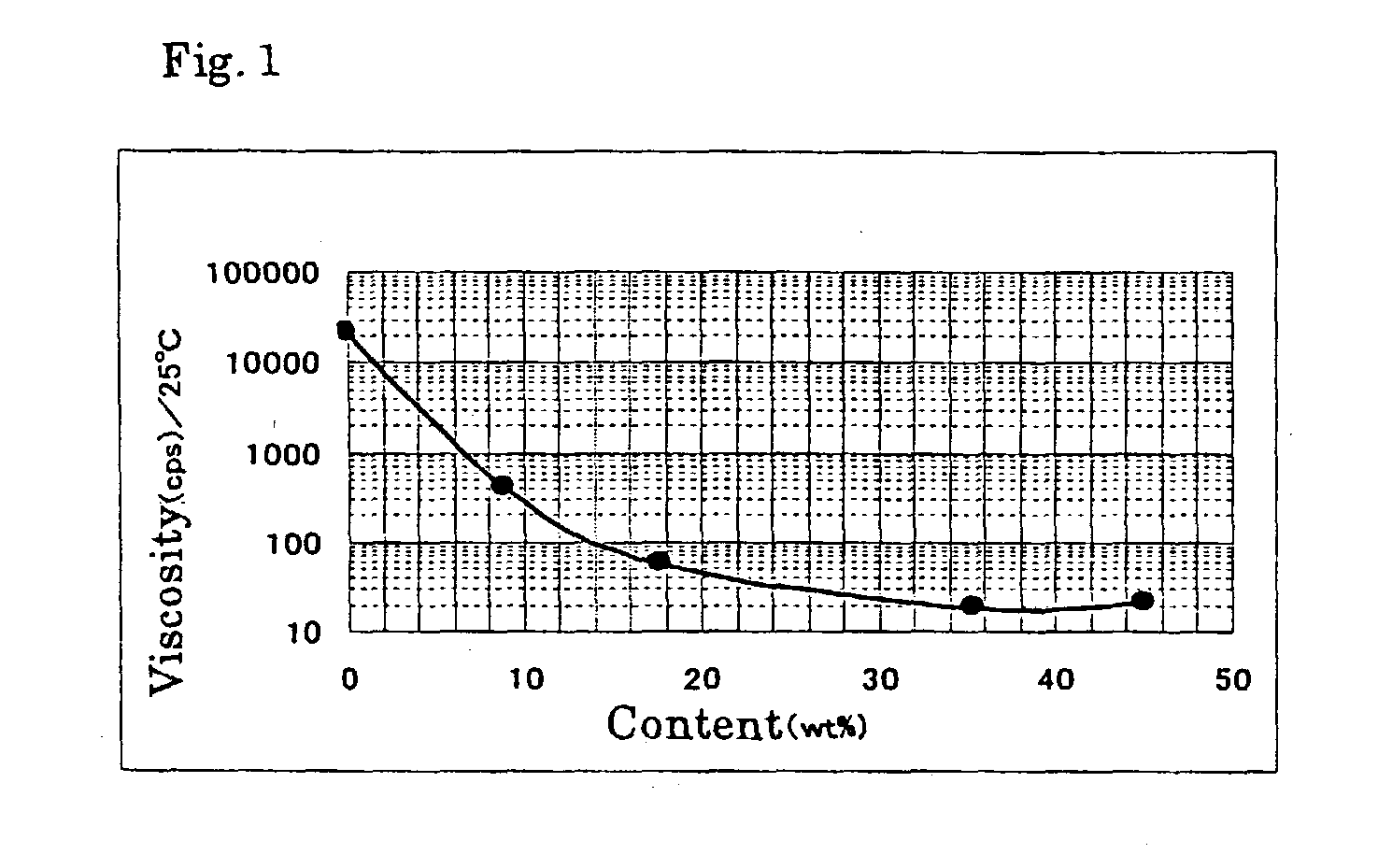
InactiveUS6858752B2Low pricePreparation from carboxylic acid halidesOrganic compound preparationCompound aPolyol
A fluorinated polyvalent carbonyl compound is produced by an economically advantageous method from inexpensive materials without requiring a complicated synthetic process step. Namely, the present invention comprises reacting a polyvalent alcohol having at least two kinds of alcohol skeletons selected among a primary alcohol, a secondary alcohol and a tertiary alcohol, with an acid halide to obtain a polyvalent ester compound, fluorinating it in a liquid phase to obtain a perfluorinated polyvalent ester compound, and cleaving the ester bonds derived from primary and secondary alcohols in the perfluoropolyvalent ester compound to obtain a fluorinated polyvalent carbonyl compound.
Owner:ASAHI GLASS CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com