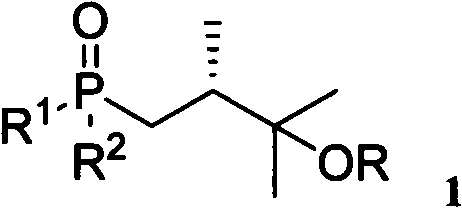
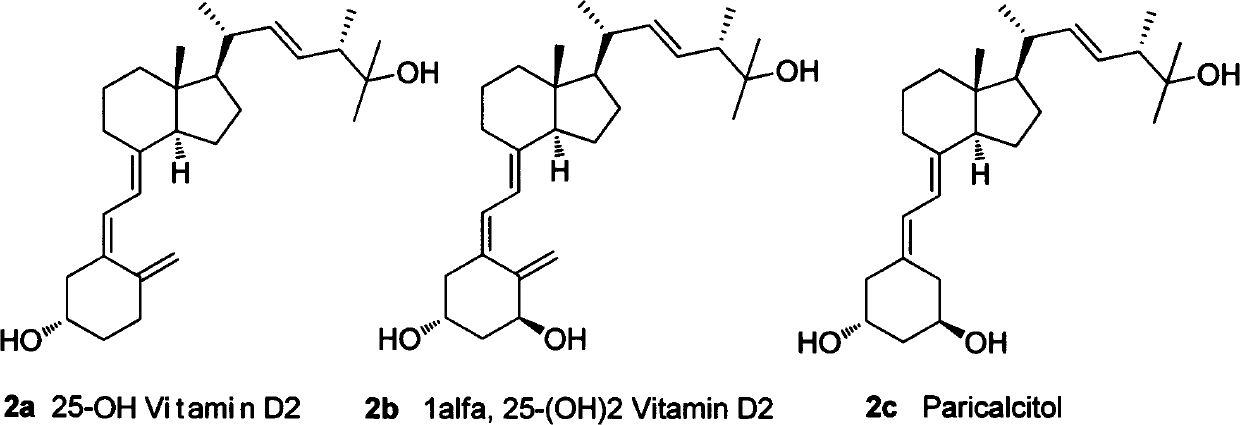
25-hydroxy vitamin D2 series medicament side chain and its preparation method
An alkyl and methyl technology, applied in the field of chain phosphorus-containing compounds and their preparation, can solve the problems of difficult synthesis and purification, inconvenient operation, large pollution, etc., and achieve the effects of short route, convenient storage and use, and wide use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: compound 1a, its molecular structure is as follows, wherein R 1 =R 2 =-OEt, R=-CH 2 OCH 3 , and its molecular structure is shown in the following formula 6:
[0033]
[0034] Formula 6
[0035] Its synthetic route is shown in the following formula 7:
[0036]
[0037] Formula 7
[0038] Dissolve (S)-2-methyl-3-hydroxypropionic acid methyl ester (11.8g, 100mmol) in diethyl ether, add MeMgBr (3M, 100mL, 300mmol) dropwise into it in an argon atmosphere at zero degrees Celsius, dropwise After that, continue to stir for 6 hours, slowly drop 1M hydrochloric acid into it to quench the reaction, extract with ethyl acetate (300mL each time) three times, combine the organic phases, dry over anhydrous magnesium sulfate, filter and concentrate to obtain colorless oil 11 (10.7g ), yield 90%.
[0039] Structure analysis data: 1H NMR (300MHz, δ, ppm) 0.81 (3H, d, J=6.9Hz), 1.14 (3H, s), 1.22 (3H, s), 1.77 (1H, m), 3.58 (1H, b), 3.66 (2H, m), 3.84 (1H, b). ...
Embodiment 2
[0046] Embodiment two: the synthesis of 1b. where R 1 = R 2 =OMe, R=THP, the molecular structure is shown in formula 8 below:
[0047]
[0048] Formula 8
[0049] Its synthetic route is shown in the following formula 9:
[0050]
[0051] Formula 9
[0052] Compound 11 (40.0 mmol) was dissolved in CH 2 Cl 2 (100mL) and pyridine (4.0g), slowly drop thionyl chloride (5.1g, 50mmol) into it under ice-salt bath cooling, continue to stir for 1 hour, after the raw material disappears, add water to quench the reaction, separate liquid, water The phase was extracted three times with dichloromethane (200 mL), dried over anhydrous sodium sulfate, concentrated by filtration and used directly in the next step.
[0053] The above chloride 13b (11.4 g, 50 mmol) was dissolved in dry CH 2 Cl 2 (100 mL), under cooling in an ice-water bath, p-toluenesulfonic acid (0.5 g) was added thereto, and then dihydropyran (5 g, 60 mmol) was slowly dropped thereinto. After reacting at room te...
Embodiment 3
[0055] Example three: Synthesis of 1c. where R 1 = R 2 =Ph, R=Bz, its molecular structure is shown in formula 10 below:
[0056]
[0057] Formula 10
[0058] Its synthetic route is shown in following formula 11:
[0059]
[0060] Formula 11
[0061] Diol 11 (11.8g) was dissolved in dry diethyl ether (100mL), and anhydrous PBr was added thereto under cooling in an ice-water bath 3 (30g), continue to stir for 1h after adding, quench with water, extract with ether, dry the organic phase with anhydrous magnesium sulfate, filter to remove desiccant, obtain iodide 13c (7g) through silica gel chromatography purification after concentration, yield 70 %.
[0062] 13c (2g) was dissolved in CH 2 Cl 2 (20mL), add pyridine (1g) and DMAP (100mg) to it, then drop benzoyl chloride (1.7g) into it, continue stirring for 2h after adding, add water to quench, after liquid separation, the organic phase is dried , after concentration, the compound 14c (2.3 g) was obtained by silica g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com