Preparation method and application of chiral tertiary alcohol or tertiary ether compound
A technology for ketone compounds and compounds is applied in the field of preparation of chiral tertiary alcohols or tertiary ether compounds, which can solve the problems of unfavorable industrial application, unstable compounds, etc., and achieves high regioselectivity and stereoselectivity, good catalysis Active, mild effect of reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
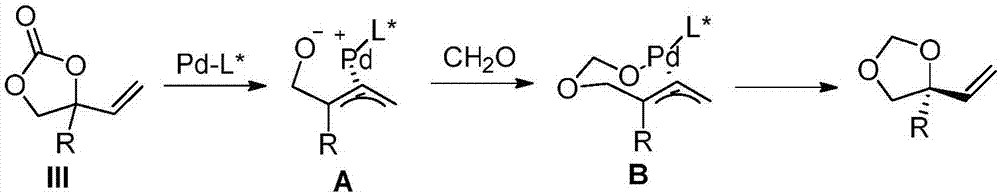
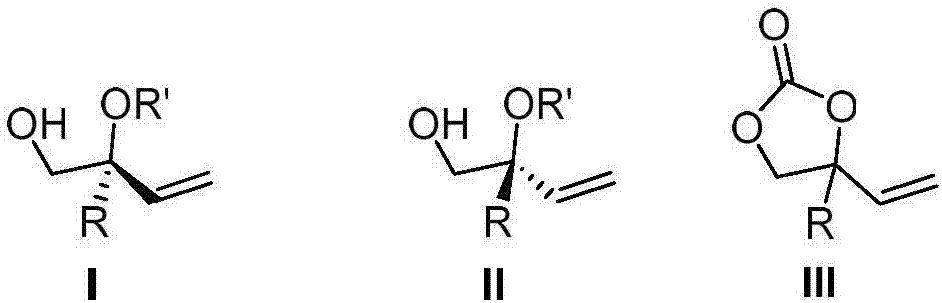
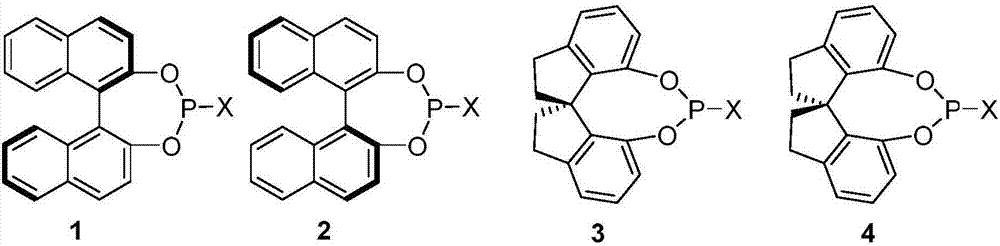
Image
Examples
Embodiment 1
[0039] This example provides the preparation of chiral tertiary alcohol Ic, in which the preparation results using different boron compounds are given.
[0040] Add Pd to the reaction tube sequentially 2 (dba) 3 CHCl 3 (0.025mmol), chiral ligand 2a[X=N(iPr)] (0.1mmol), boron compound (0.2mmol), compound IIIc (1.0mmol), water (10mmol) and tetrahydrofuran (5.0mL), 40°C The reaction was carried out for 16 hours. After the solvent was evaporated under reduced pressure, the residue was subjected to column chromatography to obtain the corresponding chiral tertiary alcohol Ic.
[0041] The reaction formula of this embodiment and the results of preparing chiral tertiary alcohol compound Ic using different boron compounds are as follows:
[0042]
Embodiment 2
[0044] This example provides the preparation of chiral tertiary alcohol compound Ic or IIc, in which the preparation results using different ligands are given.
[0045] Add Pd to the reaction tube sequentially 2 (dba) 3 CHCl 3 (0.025mmol), chiral ligand (0.1mmol), phenylboronic acid (0.2mmol), compound IIIa (1.0mmol), water (10mmol) and tetrahydrofuran (5.0mL), react at 40°C for 16 hours. After the solvent was evaporated under reduced pressure, the residue was subjected to column chromatography to obtain the corresponding chiral tertiary alcohol compound Ic or IIc.
[0046] The reaction formula of this example and the results of preparing chiral tertiary alcohol compounds Ic or IIc using different ligands are as follows:
[0047]
[0048] serial number
Embodiment 3
[0050] This example provides the preparation of chiral tertiary alcohol compound Ic or IIc, in which the preparation results using different solvents are given.
[0051] Add Pd to the reaction tube sequentially 2 (dba) 3 CHCl 3 (0.025mmol), chiral ligand 3a (0.1mmol), phenylboronic acid (0.2mmol), compound IIIc (1.0mmol), water (10mmol) and solvent (5.0mL), react at 40°C for 16 hours. After the solvent was evaporated under reduced pressure, the residue was subjected to column chromatography to obtain the corresponding chiral tertiary alcohol compound Ic or IIc.
[0052] The reaction formula of this embodiment and the results of preparing chiral tertiary alcohol compound Ic using different solvents are as follows:
[0053]
[0054] serial number
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com