Method for synthesizing alpha - olefinic bond functional cyclic carbonate from alpha ¿C alkynyl tertiary carbon alcohol and carbon dioxide
A cyclic carbonate, carbon dioxide technology, applied in the direction of organic chemistry, can solve the problems of harmfulness to human body and environment, harsh equipment requirements, non-compliance, etc., and achieve the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
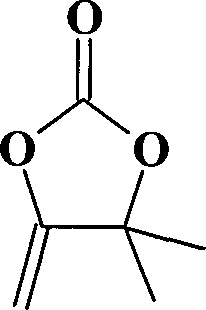
[0025] Embodiment 1: Cuprous chloride catalyzes the synthesis of α-methenyl-β, β-dimethylethylene carbonate in benzenesulfonic acid 1-methyl-3-butylimidazole ionic liquid
[0026]
[0027] In a 500ml autoclave equipped with magnetic stirring, gas valve and temperature control device, add 1-methyl-3-butylimidazole benzenesulfonate ([BMIm]PhSO 3 ) 29.6g (0.1mol) of ionic liquid, 16.8g (0.2mol) of 2-methyl-3-butyn-2-ol, 0.198g (0.002mol) of cuprous chloride, 15 kg of carbon dioxide, stirred at 120°C After reacting for 8 hours, it was cooled to room temperature and distilled under reduced pressure. The product was analyzed qualitatively and quantitatively by HP1790 gas chromatograph. The yield of α-methenyl-β,β-dimethylethylene carbonate was 97%, and the purity was 99%.
Embodiment 2
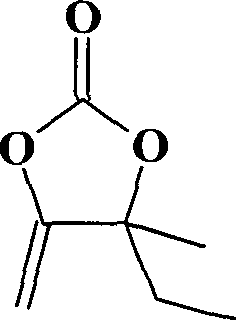
[0028] Example 2: Synthesis of α-methylenyl-β-methyl-β-ethyl ethylene carbonate catalyzed by cuprous chloride in p-toluenesulfonic acid 1-methyl-3-butylimidazolium ionic liquid
[0029]
[0030] With 31.0g (0.1mol) 1-methyl-3-butylimidazole p-toluenesulfonate ([BMIm]p-MePhSO 3 ) ionic liquid to replace [BMIm]PhSO 3 , 19.6g (0.2mol) 3-methyl-1-pentyn-3-alcohol replaces 2-methyl-3-butyn-2-alcohol, and the rest are the same as in Example 1. The yield of α-methenyl-β-methyl-β-ethyl ethylene carbonate was 96%, and the purity was 99%.
Embodiment 3
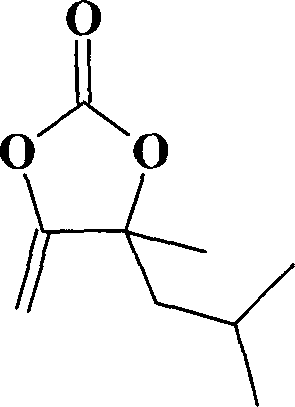
[0031] Example 3: Synthesis of α-methylenyl-β-methyl-β-isobutyl ethylene carbonate catalyzed by cuprous bromide in benzenesulfonic acid 1-methyl-3-butylimidazole ionic liquid
[0032]
[0033] Cuprous chloride is replaced by 0.287g (0.002mol) of cuprous bromide, 25.2g (0.2mol) of 3,5-dimethyl-1-hexyn-3-ol is replaced by 2-methyl-3-butyne- 2-alcohol, all the other are with embodiment 1. The yield of α-methenyl-β-methyl-β-isobutyl ethylene carbonate was 94%, and the purity was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com