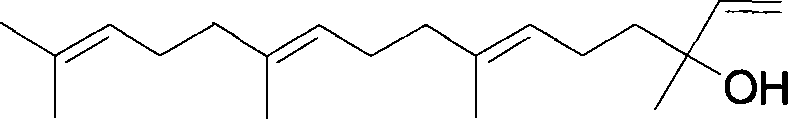
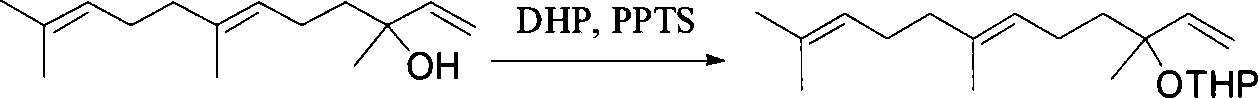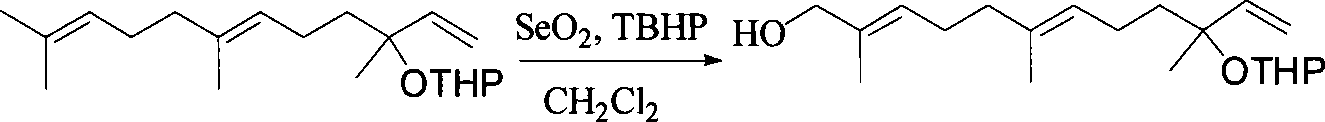Method for synthesizing (E,E) Geranyl linalool
A kind of technology of geranyl linalool and synthesis method, which is applied in the field of synthesis, can solve problems such as lack of practical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Synthesis of (E)-tertiary nerolidol tetrahydropyranyl ether
[0031]
[0032] (E)-Nerolidol (20.0g, 90mmol) and PPTS (1.58g, 3mmol) were added to dry dichloromethane (90mL), and dihydropyran (10.10g, 120mmol) was added dropwise under stirring After the dropwise addition, continue to stir overnight at room temperature, pour into a separatory funnel, add water to shake, let stand, separate the organic phase, extract the aqueous phase with dichloromethane (60mL) three times, combine the organic phases, and use anhydrous magnesium sulfate for the organic phase Dry and concentrate under reduced pressure to remove the solvent with a yield of 97%. IR and 1H NMR spectra showed that the structure of the product was correct.
[0033] 2) (E, E)-12-hydroxynerolidol tetrahydropyranyl ether
[0034]
[0035] SeO2 (0.666g, 6mmol) and 75% t-BuOOH (7.2g, 60mmol) were dissolved in 30mL of CH2Cl2, slowly added dropwise with CH2Cl2 solution (40mL) of geraniol tetrahydropyranyl e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com