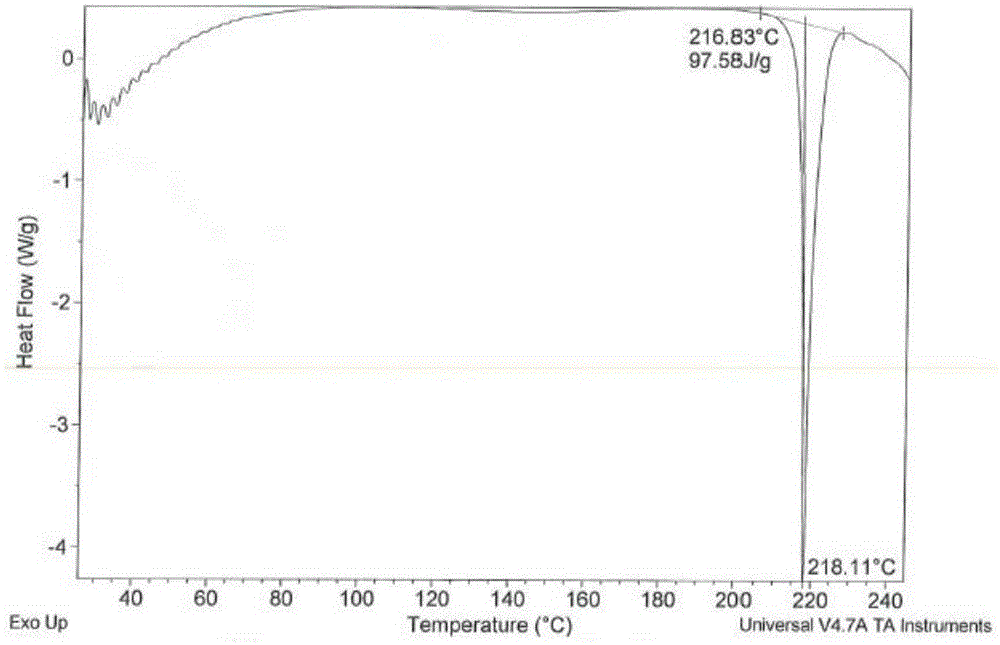
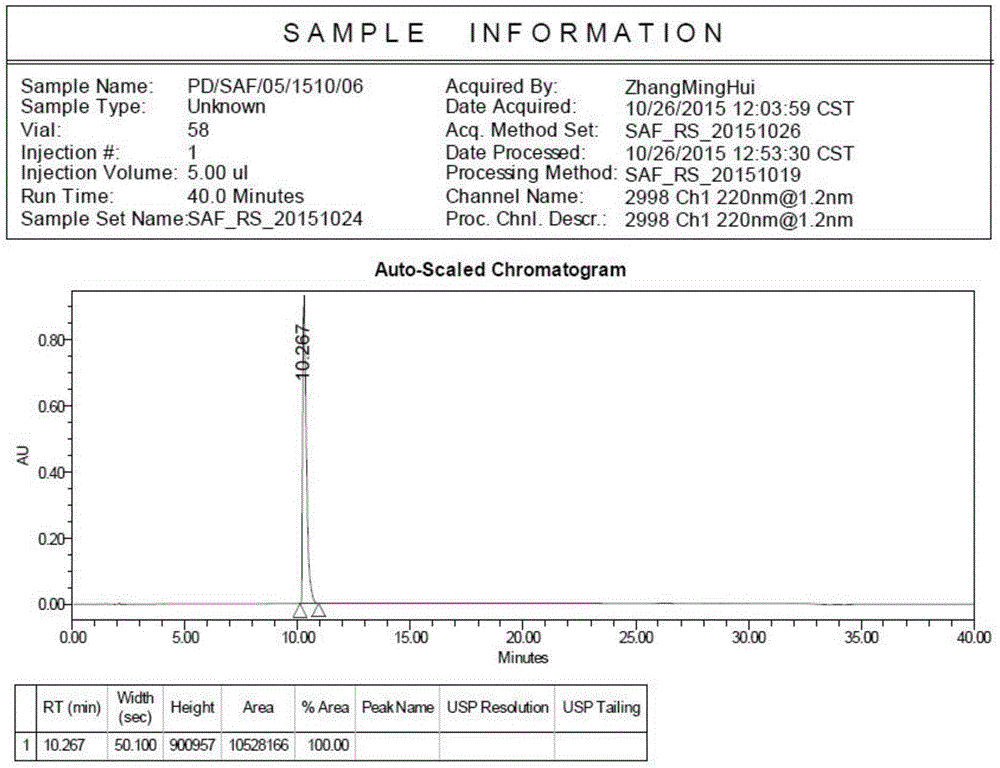
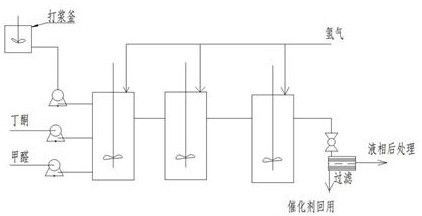
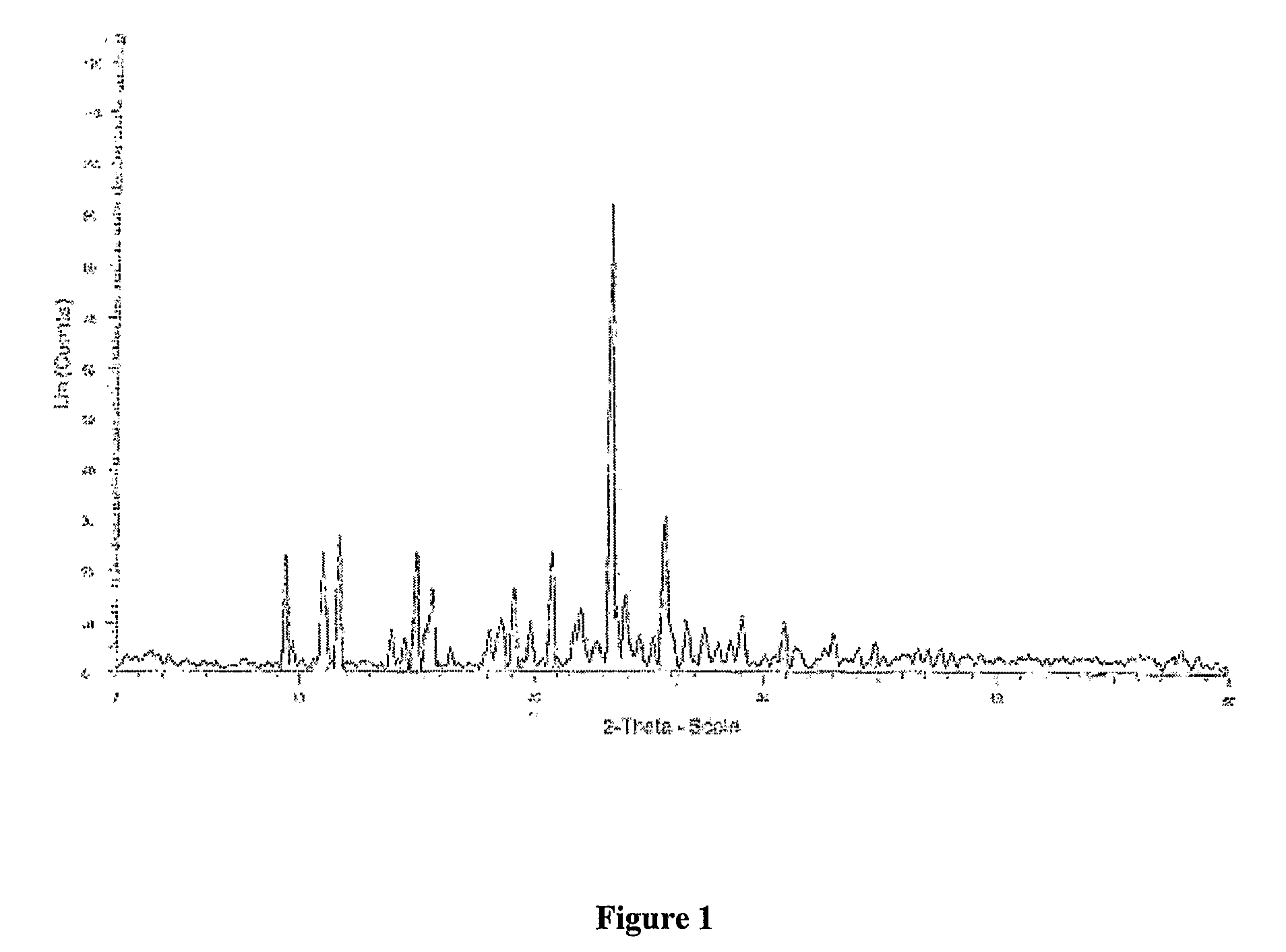
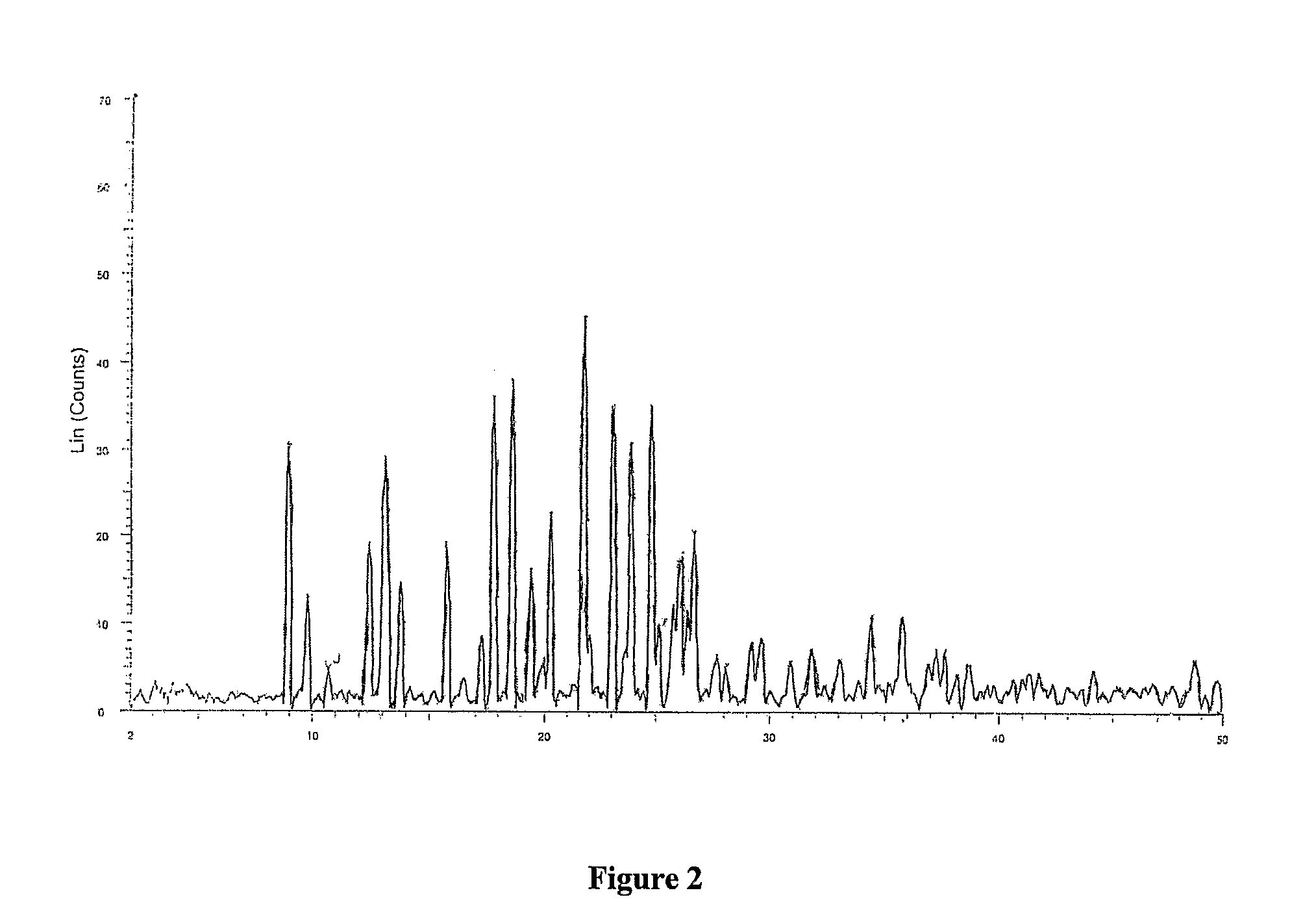
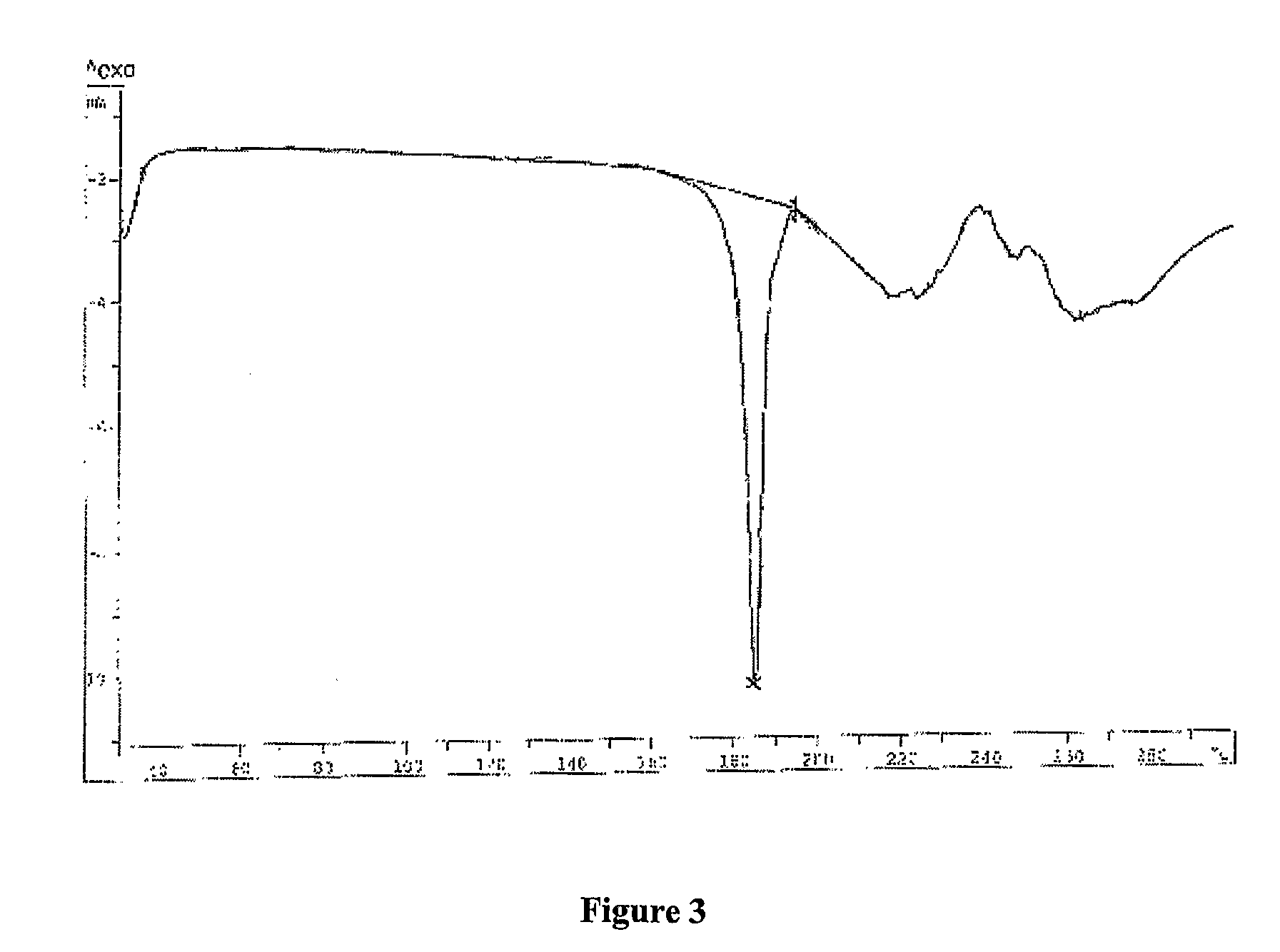
Patents
Literature
80 results about "Isopropyl methyl ketone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
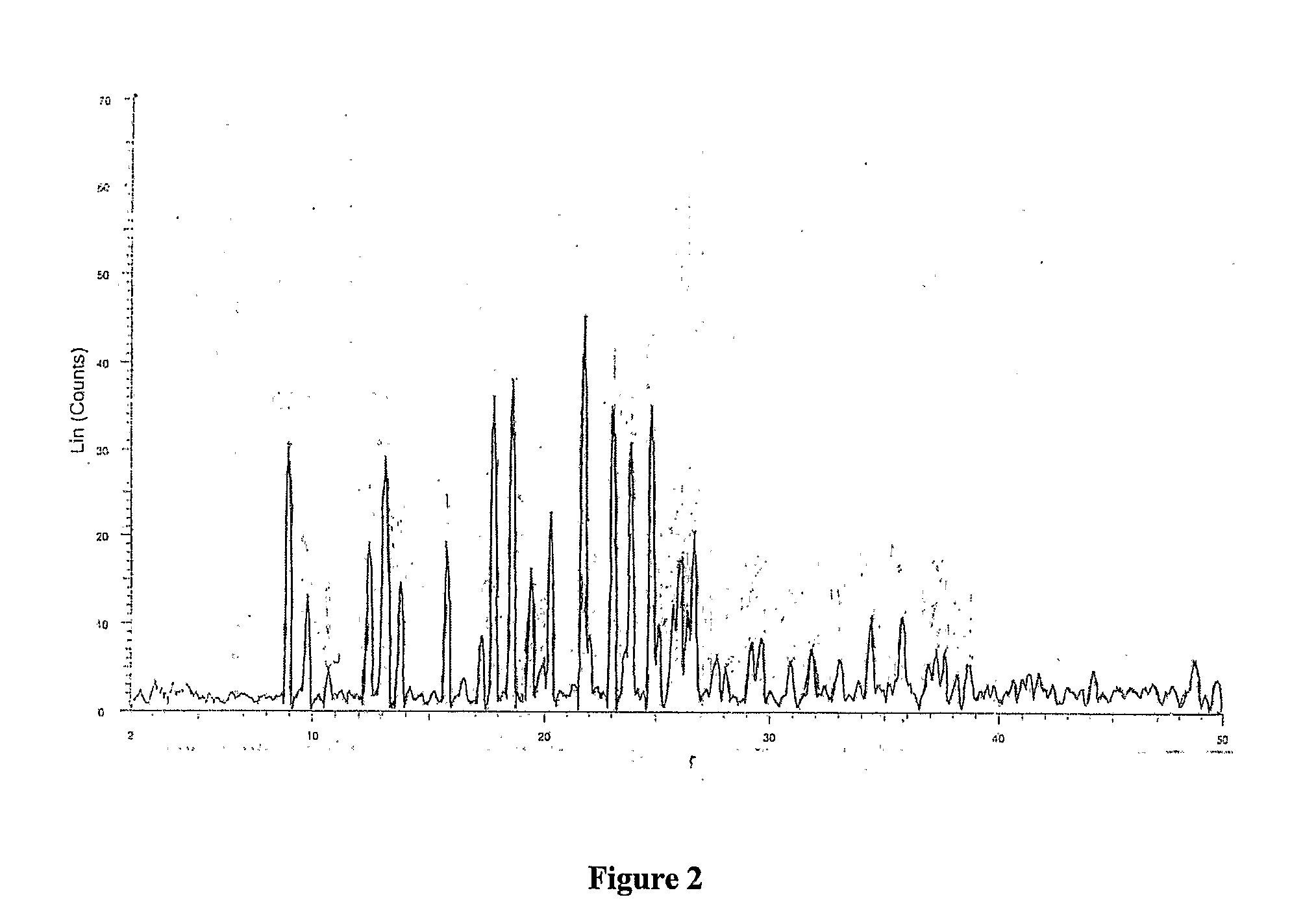
3-Methyl-2-butanone (methyl isopropyl ketone, MIPK) is a ketone and solvent of minor importance. It is comparable to MEK (Methyl ethyl ketone), but has a lower solvency and is more expensive. "NIOSH Pocket Guide to Chemical Hazards #0424". National Institute for Occupational Safety and Health (NIOSH).
Method for separating lignose and cellulose in licorice waste slag by means of ultrasonic technology
The invention relates to a method for separating lignin and cellulose from the wastes generated during the processes of extracting flavone, glycyrrhizic acid or polyoses from the traditional Chinese herb of liquorice. The method includes the following steps: (1) the waste residue of liquorice is added into the mixed solvent of methyl isopropyl ketone-ethanol-water and is manufactured into a turbid liquid; (2) the turbid liquid is treated by ultrasonic and then is stood for a certain period of time and filtered to obtain the filtrate and filter residues; (3) after the filtrate is condensed in vacuum and the solvent is recycled, a crystal is obtained; then the obtained filtrate is filtered and dried to obtain the lignin; the filter residues are directly dried to obtain the cellulose. The method for separating lignin and cellulose from the waste residue of the liquorice by utilizing the ultrasonic technology can overcome the defects of environment pollution and resource wasting caused by a soda boiling method and sulphite as well as the defect of high energy consumption caused by a high-boiling alcohol solvent method. The method creates a new approach for the comprehensive utilization of the waste residues of the liquorice.
Owner:TIANJIN UNIV OF SCI & TECH
Method for preparing polyester containing isosorbide
The invention discloses a method for preparing a polyester containing isosorbide, comprising the following steps of: carrying out dehydration reaction on raw materials including isosorbide and dicarboxylic acid which have equal molar weight under the action of a polycondensation catalyst and a water-carrying agent under normal pressure, and carrying out a vacuum polycondensation to prepare the polyester containing isosorbide, wherein the water-carrying agent comprises one or more of the methyl isopropyl ketone, ethyl isopropyl ketone, methyl isobutyl ketone, diisopropyl ketone and diisobutyl ketone. According to the method, an appropriate amount of water-carrying agent is added during the direct esterification reaction of the isosorbide and the binary acid, thus the reaction temperature is decreased greatly, and the molecular weight and the polymerization efficiency of the polyester are improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
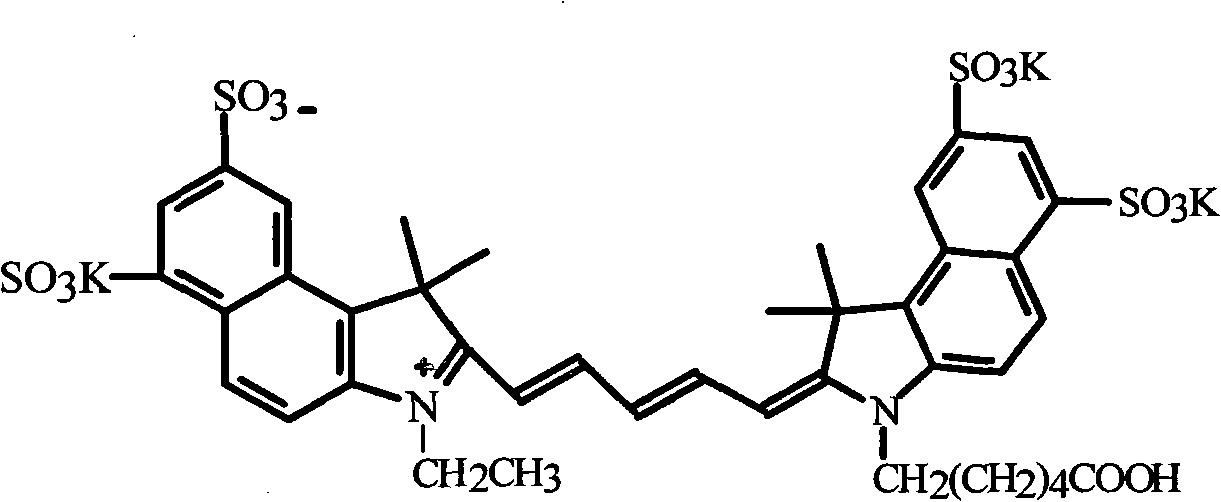
Method for preparing and purifying water-soluble cyanine dye (Cy5.5)
ActiveCN102010614AEasy to operateLow costMethine/polymethine dyesMethyl groupThin layer chromatogram
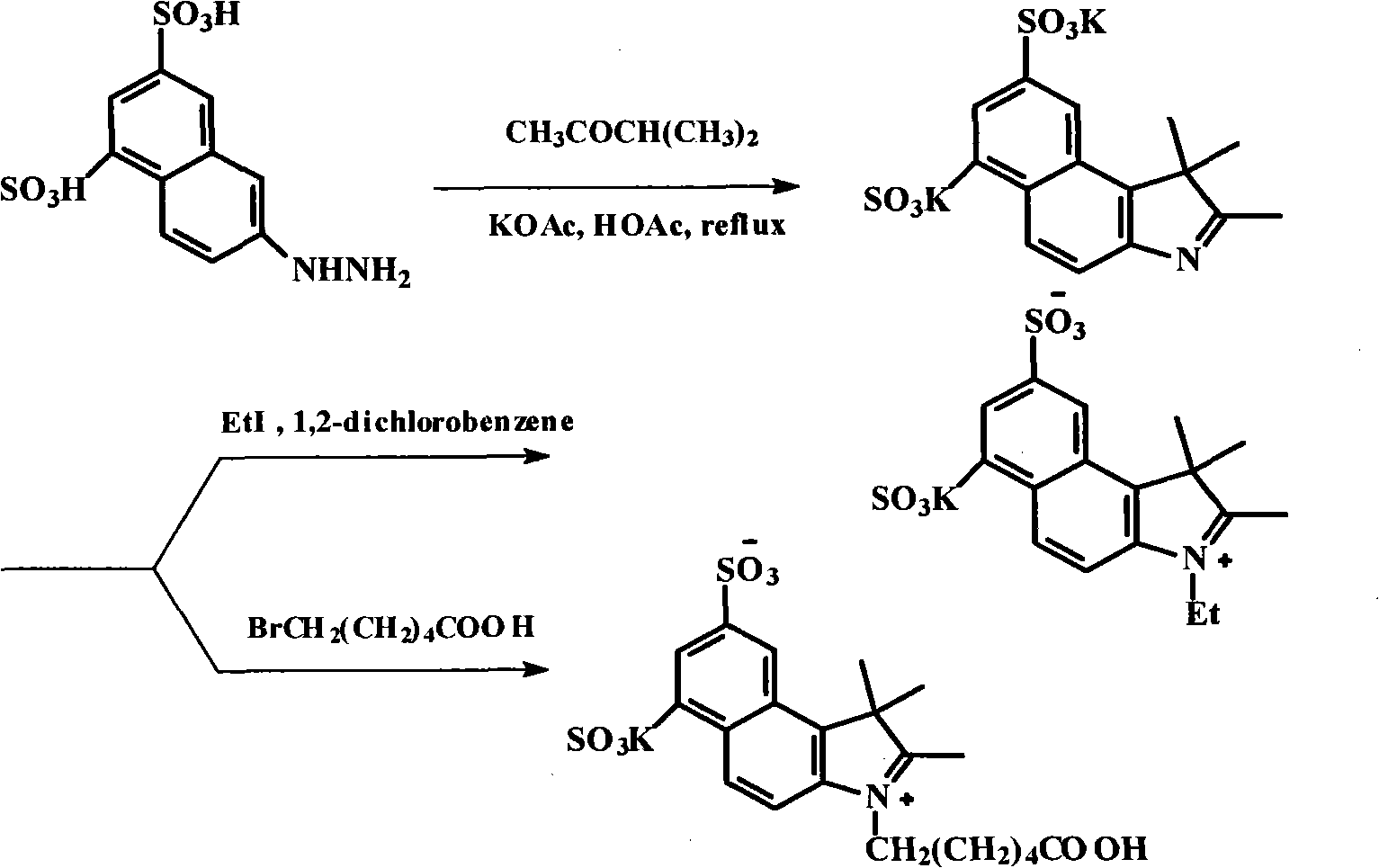
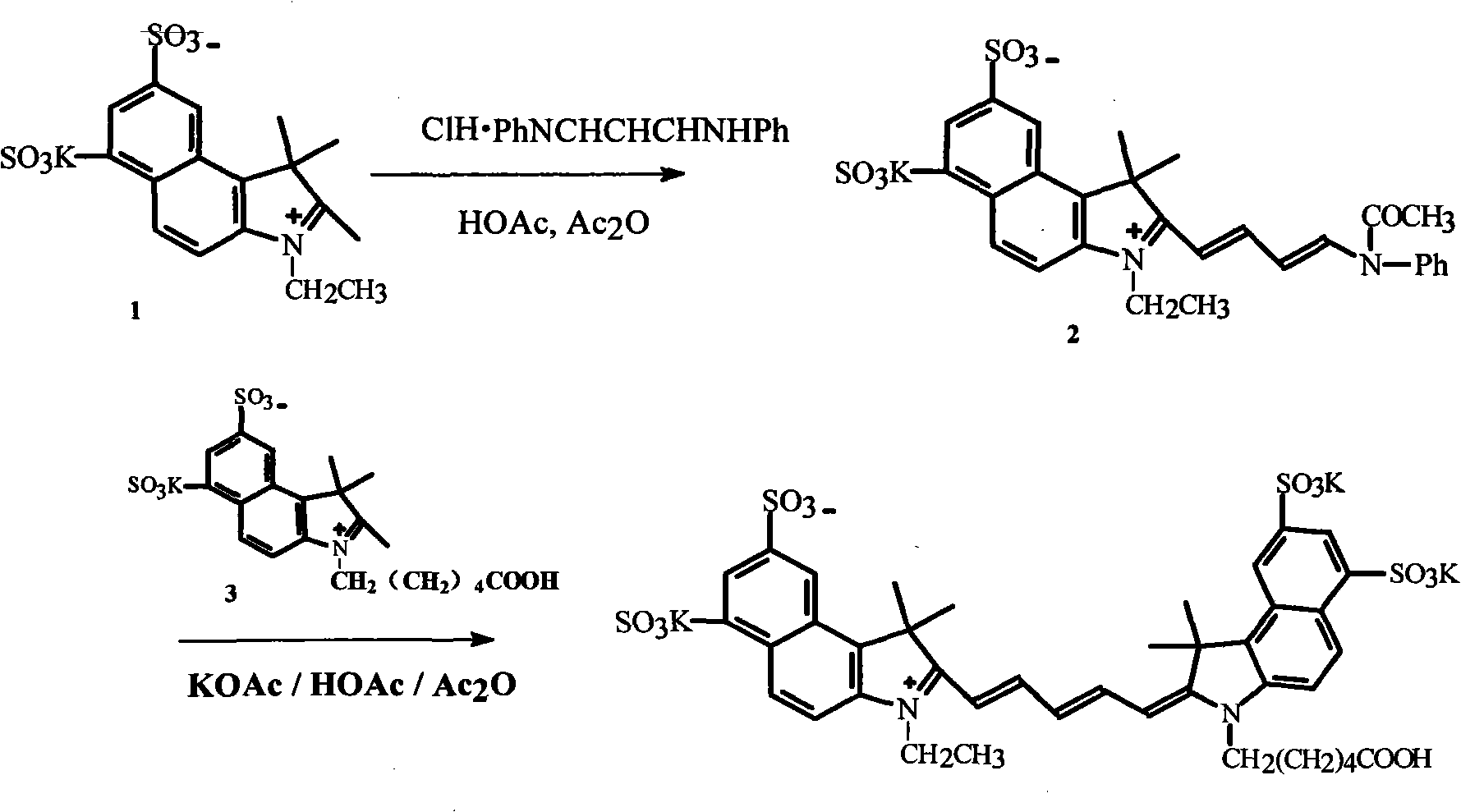
The invention discloses a method for preparing and purifying a water-soluble cyanine dye (Cy5.5), which comprises the following steps of: reacting 1,3-disulfonic acid-6-naphthylhydrazine serving as an initiative raw material with methyl isopropyl ketone to generate 1,1,2-trimethyl-benzindole-1,3-disulphonate; reacting the 1,1,2-trimethyl-benzo-indole-1,3-disulfonate with ethyl iodide and 6-bromocaproic acid respectively to generate N-ethyl-2,3,3-trimethyl-benzindole-5,7-disulphonate (product 1) and N-(epsilon-carboxy-pentyl)-2,3,3-trimethyl-benzindole-5,7-disulphonate (product 2); reacting the product 1 with beta-anilino-acraldehyde-anil-hydrochloride and then directly reacting the product 1 with the product 2 to prepare the Cy5.5; and preparing a thin layer chromatogram, and repeatedly purifying and separating the Cy5.5 to obtain a pure product of the Cy5.5. The method is easy and convenient to operate and has a high yield, the product does not need expensive high performance liquid chromatography (HPLC) (C-18 reverse column) for separation, and the method has great significance for meeting requirements of domestic autonomous production.
Owner:TIANJIN SUNGENE BIOTECH
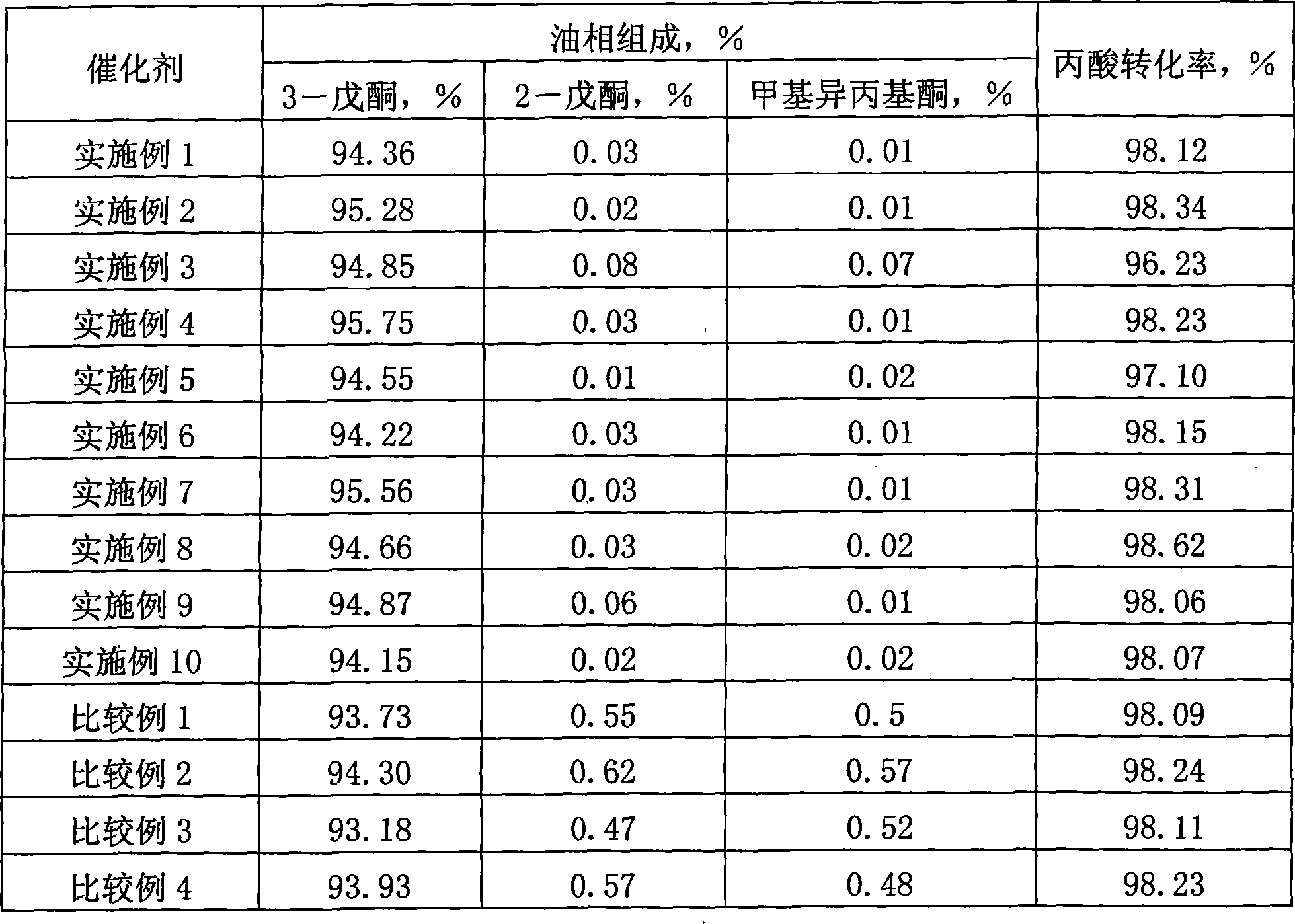
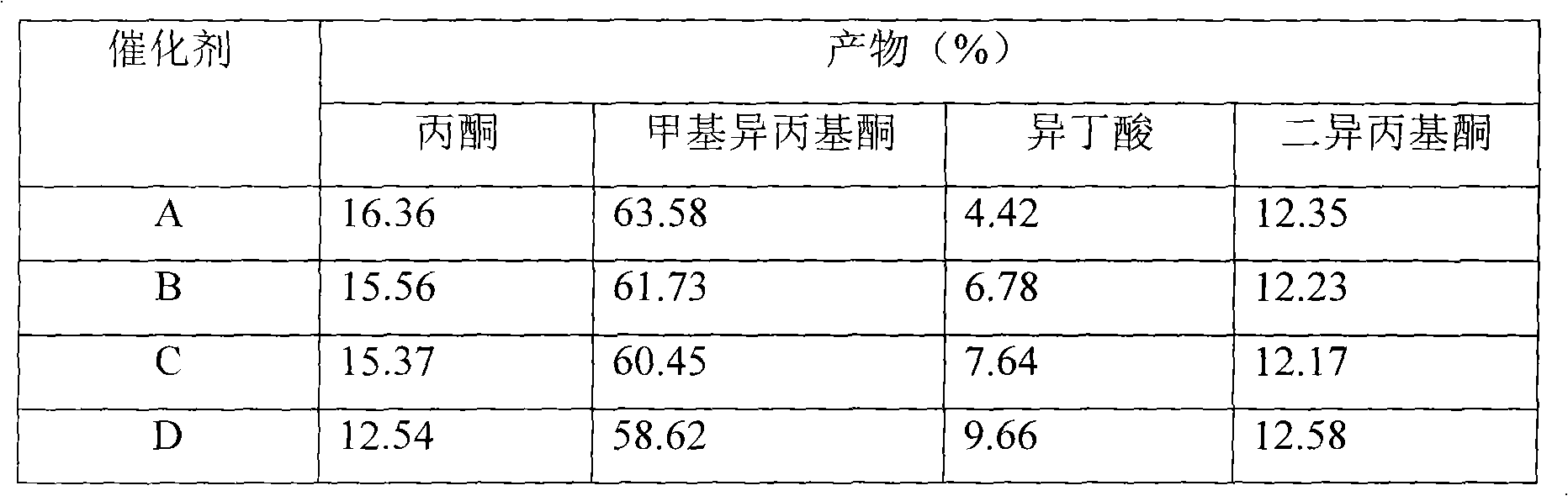
Catalyst for synthesizing 3-pentanone
InactiveCN101507919AReduce the difficulty of separationKeep aliveOrganic compound preparationCarbonyl compound preparationIsomerizationRare earth
The invention relates to a catalyst for synthesizing 3-pentanone, and belongs to the technical field of preparation of catalysts for fine chemical products. The catalyst consists of an active composition, an auxiliary agent and a carrier, wherein the active composition is one or more of lanthanum rare earth oxide and actinium rare earth oxide; the carrier is one or more of zirconia, alumina, titania and silicon dioxide; the weight ratio of the active composition to the carrier is 5-80:95-20; and the auxiliary agent is one or more alkali metals or alkaline earth oxides and is 0.1 to 5 weight percent of the total weight of the active composition and the carrier. The catalyst not only maintains the activity of the prior catalyst, but also overcomes the defects of the prior rare earth catalyst, achieves the aims of inhibiting the isomerization reaction and reducing the content of 2-pentanone and methyl isopropyl ketone in the reaction products, and greatly reduces the separation difficulty of the 3-pentanone.
Owner:YIXING ZHONGGANG FINE CHEM
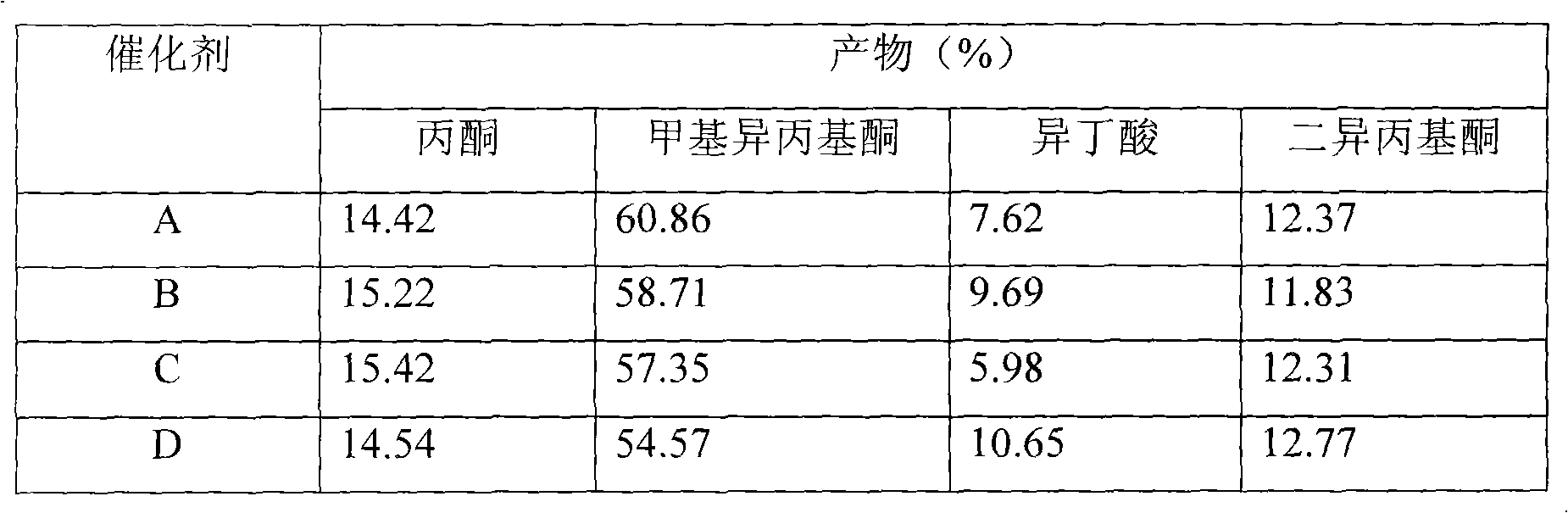
Catalyst for synthesizing methyl isopropyl ketone and diethylketone, process for preparing the same and application thereof
InactiveCN1733360AHigh activityIncrease productivityOrganic compound preparationCarbonyl compound preparationMixed oxideMetallole
This invention provides new catalyst with high activity to synthesize methyl isopropenyl ketone and ethanone from MEK and carbinol, its preparation method and application in ketone preparation reaction. The catalyst comprises Zr, Mn, Zn and mixed oxide of alkali metal carried a little Pd. By this product, the reaction can run at very low temperature, does not coke easily, needs less raw material and the cost is lower.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
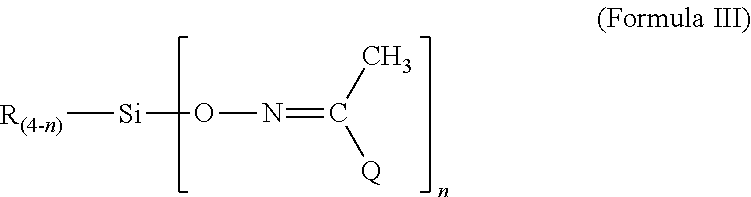
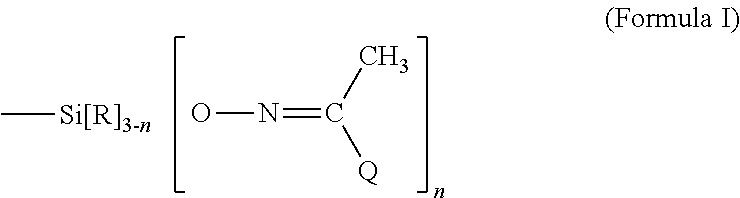
Room temperature vulcanizable polymers
ActiveUS20110028639A1Good optical performanceShort durationSilicon organic compoundsRoom temperaturePolymer
A room-temperature vulanizable (RTV) polymeric composition comprising organosiloxane or polyurethane units having at least one terminal methyl isopropyl ketoximino or methyl propyl ketoximino moiety, as well as methods of making the same.
Owner:NITROCHEM ASCHAU
Method for synthesizing methyl isopropyl ketone
InactiveCN102049251ACarbonyl compound preparation by condensationMetal/metal-oxides/metal-hydroxide catalystsRare-earth elementGas phase
The invention relates to a method for synthesizing methyl isopropyl ketone, which is characterized in that rare earth oxides are loaded on an aluminum oxide, titanium oxide or titanium-aluminum compound carrier to be used as a synthesis catalyst, wherein rare earth elements in the rare earth oxides are lanthanum, cerium, praseodymium and neodymium, the loading capacity of the rare earth metals is 3-20%, and the molar ratio of titanium to aluminum in the carrier is 0.2-0.5. A preparation method of the catalyst comprises the following steps of: weighing a certain amount of nitrates of rare earth metal oxides to prepare a uniform solution; then, adding a certain amount aluminum oxide, titanium oxide or titanium-aluminum compound carrier into the solution, and impregnating for 5-10h at 25 DEG C; vacuumizing and drying for 3-6h at 120 DEG C, and roasting for 4-8h at 450-700 DEG C in a muffle furnace to obtain the catalyst. Methyl isopropyl ketone is synthesized by using isobutyric acid and acetic acid as synthesis raw materials under the conditions that the reaction temperature is 420-440 DEG C and the liquid hourly space velocity is 0.5-0.8 g.g<-1>.h<-1>, and the molar ratio of isobutyric acid to acetic acid is 0.8-1.2:1. The reaction process is as follows: a raw material mixture is pumped into a fixed bed reactor through a charging pump, and the content of the products is analyzed by using gas chromatography after the raw material mixture reacts in a catalyst bed.
Owner:CHINA NAT OFFSHORE OIL CORP +1
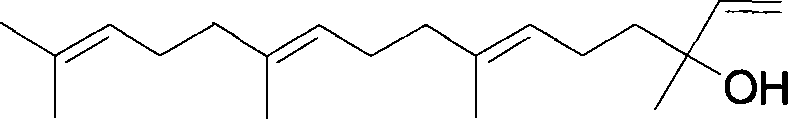
Method for synthesizing (E,E) Geranyl linalool
InactiveCN101070270AOrganic compound preparationHydroxy compound preparationSulfonyl chlorideNerolidol
This invention relates to synthetic method of a ( E, E) - geranyl linalool. The invention takes (E) - nerolidol as raw material. The hydroxyl is shield by dihydropyrane, gain ( E) - nerolidol tetrahydropyrane aether; selenium dioxide and teri-butyl hydroperoxide selectively oxidize the anti-form methyl of ( E) - nerolidol tetrahydropyrane aether to gain anti-form allyl position hydroxylated oxidative product ( E, E) - 12 - hydroxy nerolidol tetrahydropyrane aether, transit halogenating reaction to gain ( E, E) - 12 - halogeno- nerolidol tetrahydropyrane aether, then take reaction with isopropyl methyl ketone that is selectively divested one proton by diisopropyl amido lithium, generate ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - ketone, use sodium borohydride to reduce to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 - oxygen) -16 - 6, 10, 15 - triene - 3 - alcohol, takes reaction with sulfonyl chloride or sulphonic acid ester with alkali presence to gain ( 6E, 10E) - 2, 6, 10, 14 - tetramethyl - 14 - ( tetrahydropyrane - 2 oxygen) -16 - 6, 10, 15 - triene - 3 - alcoholic sulphonic acid ester, then divide sulphonic acid ester group under base catalysis to gain ( E, E) - geranyl linalool tetrahydropyrane aether, and by deprotection to gain ( E, E) - geranyl linalool. ËFor the configuration of ( E) - nerolidol 3 position tertiary carbon is not influenced in the course of reaction, if use ( E) - nerolidol that has optical activity as raw material, should gain optical active ( E, E) - geranyl linalool. ((E, E)-geranyl linalool can replace Teprenone and such type medicament intermediate, natural product intermediate, insect pheromone and spice etc.
Owner:CHENGDU UNIVERSITY OF TECHNOLOGY
Room temperature vulcanizable polymers
ActiveUS8609797B2Good optical performanceSilicon organic compoundsRoom temperatureIsopropyl methyl ketone
A room-temperature vulanizable (RTV) polymeric composition comprising organosiloxane or polyurethane units having at least one terminal methyl isopropyl ketoximino or methyl propyl ketoximino moiety, as well as methods of making the same.
Owner:NITROCHEM ASCHAU
Method for producing methyl isopropyl ketone
InactiveCN101168497AKeep aliveImprove stabilityCarbonyl compound preparation by condensationIsomerizationIsopropylacetone
The invention relates to a process for producing methyl isopropylacetone, which uses H3PO4-B / bergmeal as catalyst, converts isoprene and water in a thermal-insulation reactor into methyl isopropylacetone. The process comprises that gasified water and isoprene are preheated, to be fed into a catalyst bed via the top of the reactor to react, wherein the phosphate content of the catalyst is 50-80% of catalyst mass, while the boron content is 4-9%. When the reaction temperature is 180-300DEG C, the mol ratio between water and isoprene is 4.0-8.0, air-speed LHSV is 0.1-0.5h-1, and the reaction pressure is normal pressure as 0.3MPa, isoprene and water via addition and isomerization generate methyl isopropylacetone. Compared with prior H3PO4 bergmeal catalyst, the invention uses H3PO4-B bergmeal catalyst to improve catalyst stability and effectively overcome the defects of H3PO4-B bergmeal catalyst as intrinsic easy phosphate loss and catalyst pelitization, to prolong the service life of catalyst, thereby reducing the catalyst application cost and production cost of methyl isopropylacetone.
Owner:胡建国
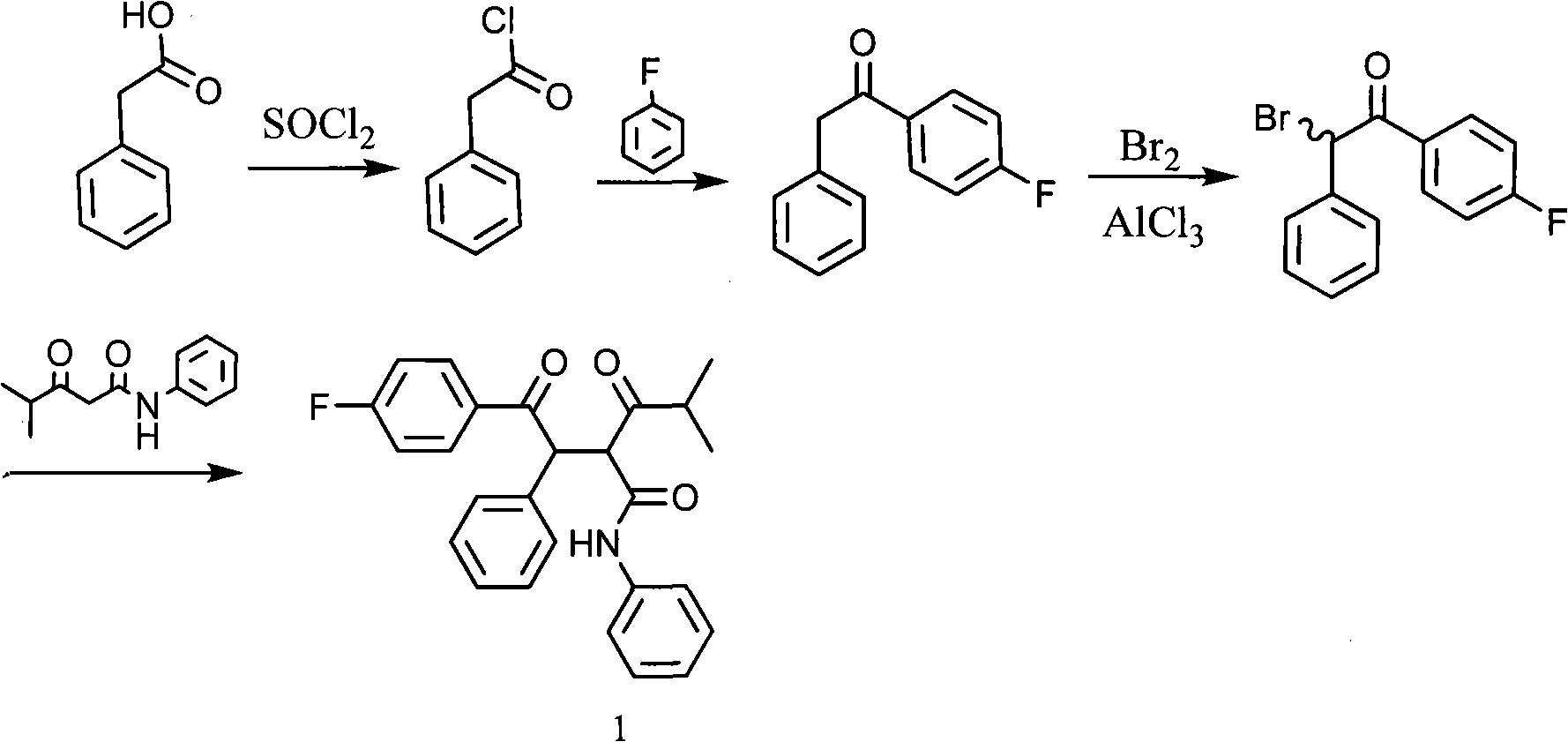
New synthetic method for (earth)4-fluor-alpha-(2-methyl-1-oxypropyl )-gamma-oxo-N, beta-diphenyl benzene butanamide
InactiveCN101307009ALow costHigh yieldOrganic compound preparationCarboxylic acid amides preparationAluminium chlorideMethyl carbonate
The invention discloses a method for preparing an intermediate product of an Atorvastatin medicine. The isobutyrylacetanilide utilized in the prior method has low yield coefficient, expensive raw materials and high industrial cost. The method comprises the following steps that: 4- fluorophenyl benzyl ketone is obtained by performing the Friedel-Crafts reaction on phenylacetyl chloride and fluorobenzene which are catalyzed by aluminium chloride; methyl isopropyl ketone reacts with dimethyl carbonate or diethyl carbonate in an inert solvent with sodium hydrosulfate as catalyst to produce methyl 4-methyl-3-Oxopentanoate or ethyl isobutyrylacetate; next, the methyl 4-methyl-3-Oxopentanoate or the ethyl isobutyrylacetate reacts with aniline to produce isobutyrylacetanilide; carbon-based alpha position of the 4-fluorophenyl benzyl ketone is chloridized in the solvent to produce alpha-chlorine-4-fluorophenyl benzyl ketone; afterwards, the alpha-chlorine-4-fluorophenyl benzyl ketone reacts with the isobutyrylacetanilide in the action of alkali. The process for synthesizing isobutyrylacetanilide has short flow, the yield coefficient is high, and the cost is low, thereby improving the total yield of reaction directly.
Owner:ZHEJIANG JINGXIN PHARMA
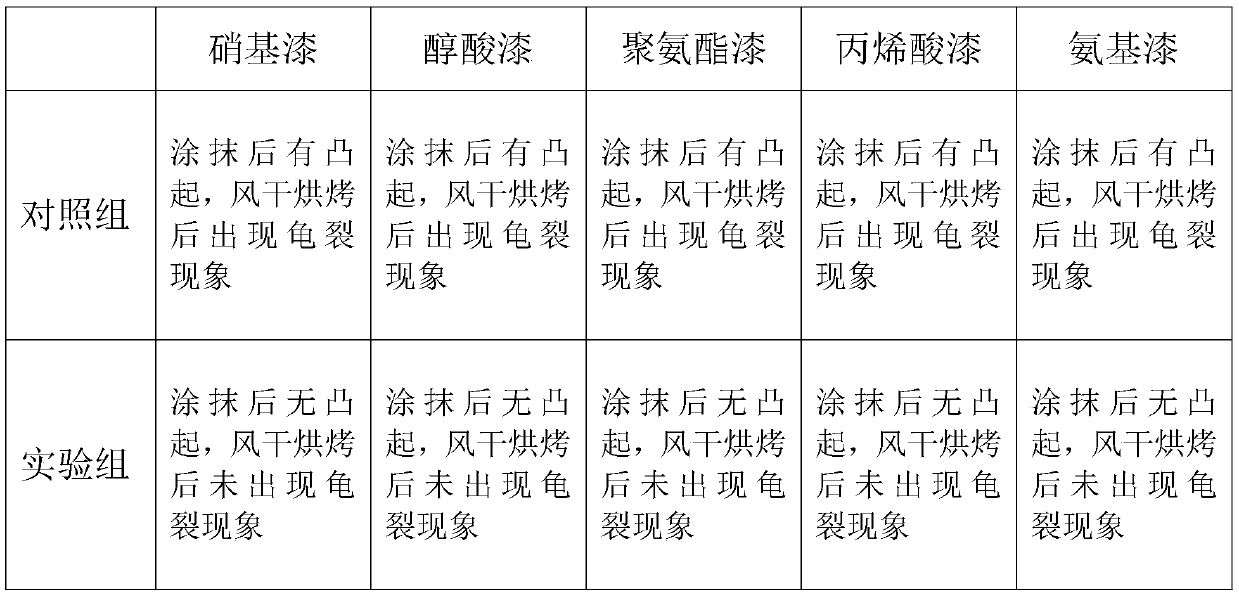
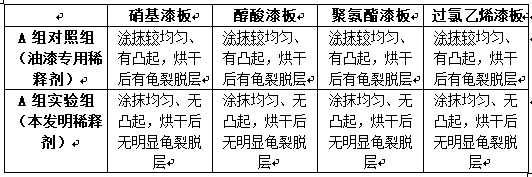
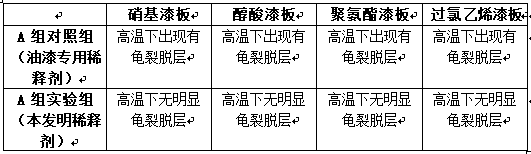
Multi-purpose paint diluting agent and preparation technology thereof
The invention discloses a multi-purpose paint diluting agent and a preparation technology thereof. The multi-purpose paint diluting agent is prepared from the following raw materials: ethylene glycolmonoethyl ether acetate, dichloropropane, pentaerythritol, cyclohexanone, tetrabutyl orthosilicate, ethyl cellosolve, diethoxymethane, methyl isopropyl ketone, turpentine and isophoron. The paint diluting agent prepared by the preparation technology does not contain benzene toxic substances, is safe and has no toxin, and has good stability and strong dissolubility; various types of paint can be dissolved through the multi-purpose paint diluting agent; the production flow is simple, the production cost is low and no three wastes are discharged, so that the multi-purpose paint diluting agent hasa good application prospect.
Owner:韩立志
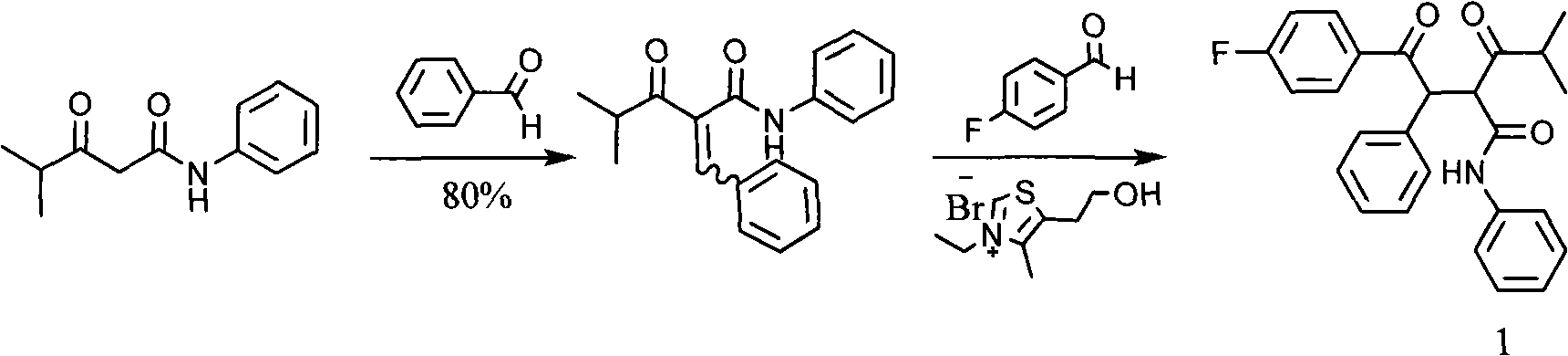
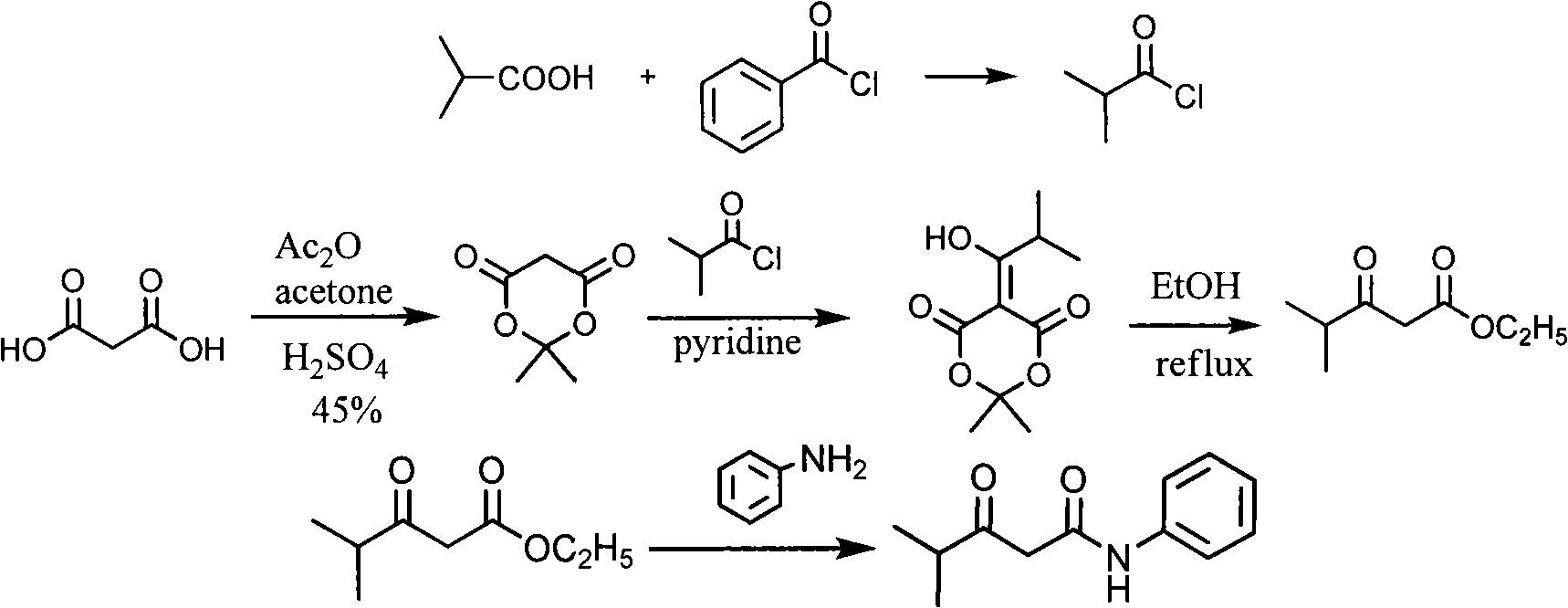
Synthesis method of 2-[2-(4-fluorophenyl)-2-oxa-1-phenylethyl]-4-methyl-3-oxa-N-phenylpentanamide
InactiveCN102382006AEasy to operateLow costOrganic compound preparationCarboxylic acid amides preparationSynthesis methodsAcetophenone
The invention discloses a synthesis method of 2-[2-(4-fluorophenyl)-2-oxa-1-phenylethyl]-4-methyl-3-oxa-N-phenylpentanamide. The method is characterized by comprising the following steps: firstly preparing methyl isobutyryl acetate from toluene, sodium hydride, dimethyl cabonate, methyl isopropyl ketone and the like as raw materials; then preparing isobutyryl acetanilide from toluene, methyl isobutyl acetate, HCl, sodium bicarbonate and the like as raw materials; preparing 4-fluorophenylacetophenone from aluminium choride, phenylacetyl chloride and the like as raw materials; preparing 2-chloro-1-(4-fluorophenyl)-acetophenone from 4-fluorophenylacetophenone, dichloromethane, chlorine and the like as raw materials; and finally preparing the finished product from isobutyryl acetanilide, potassium carbonate and 2-chloro-1-(4-fluorophenyl)-acetophenone. The raw materials of the synthesis method are inexpensive and easily available, the reaction has five steps and the yield of each step is high, thus the production cost can be greatly reduced; and the reaction conditions are mild, the method can be controlled easily, the process is simple, the pollution is low and the method is suitable for industrial production.
Owner:LIANYUNGANG HONGYE CHEM
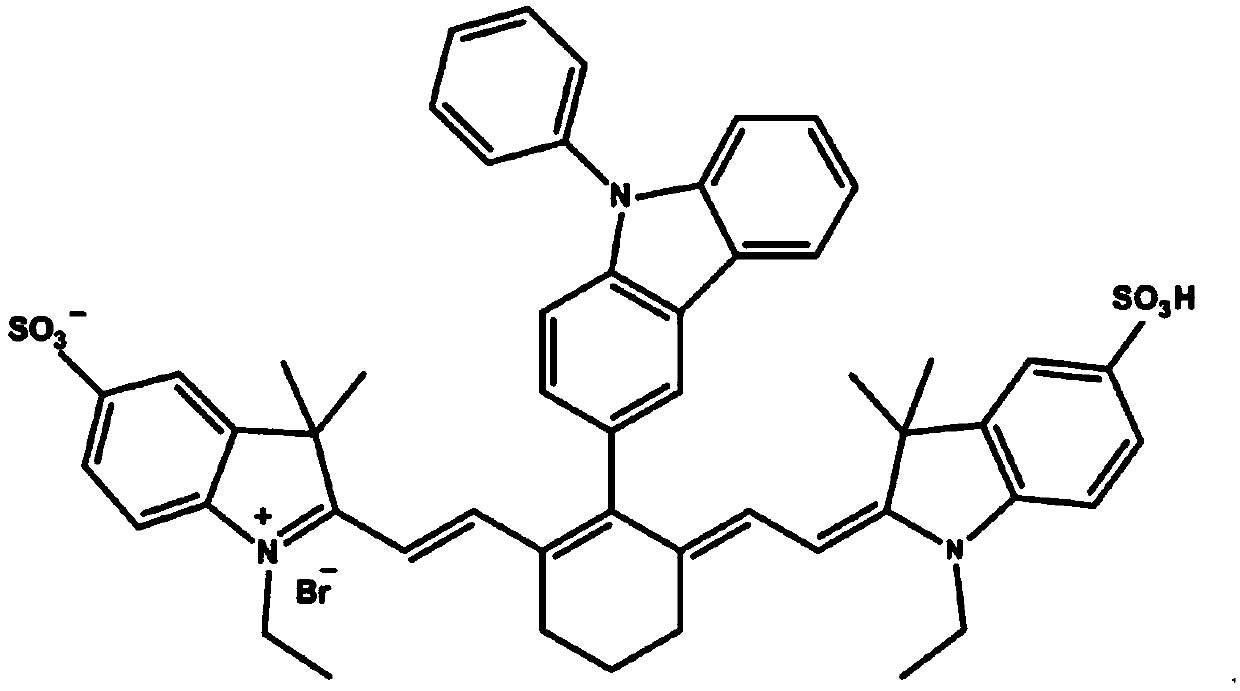
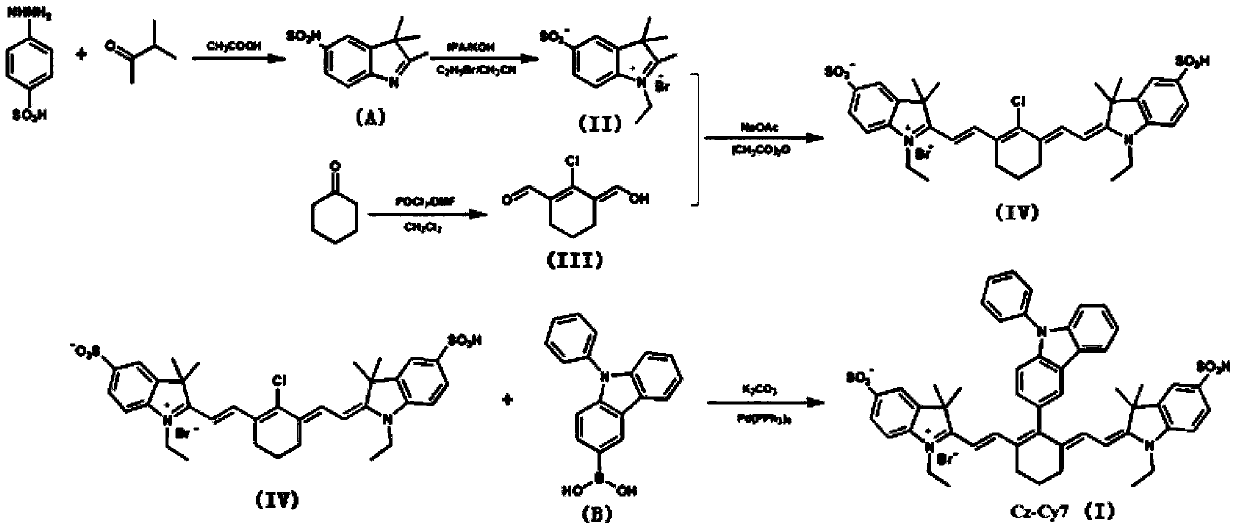
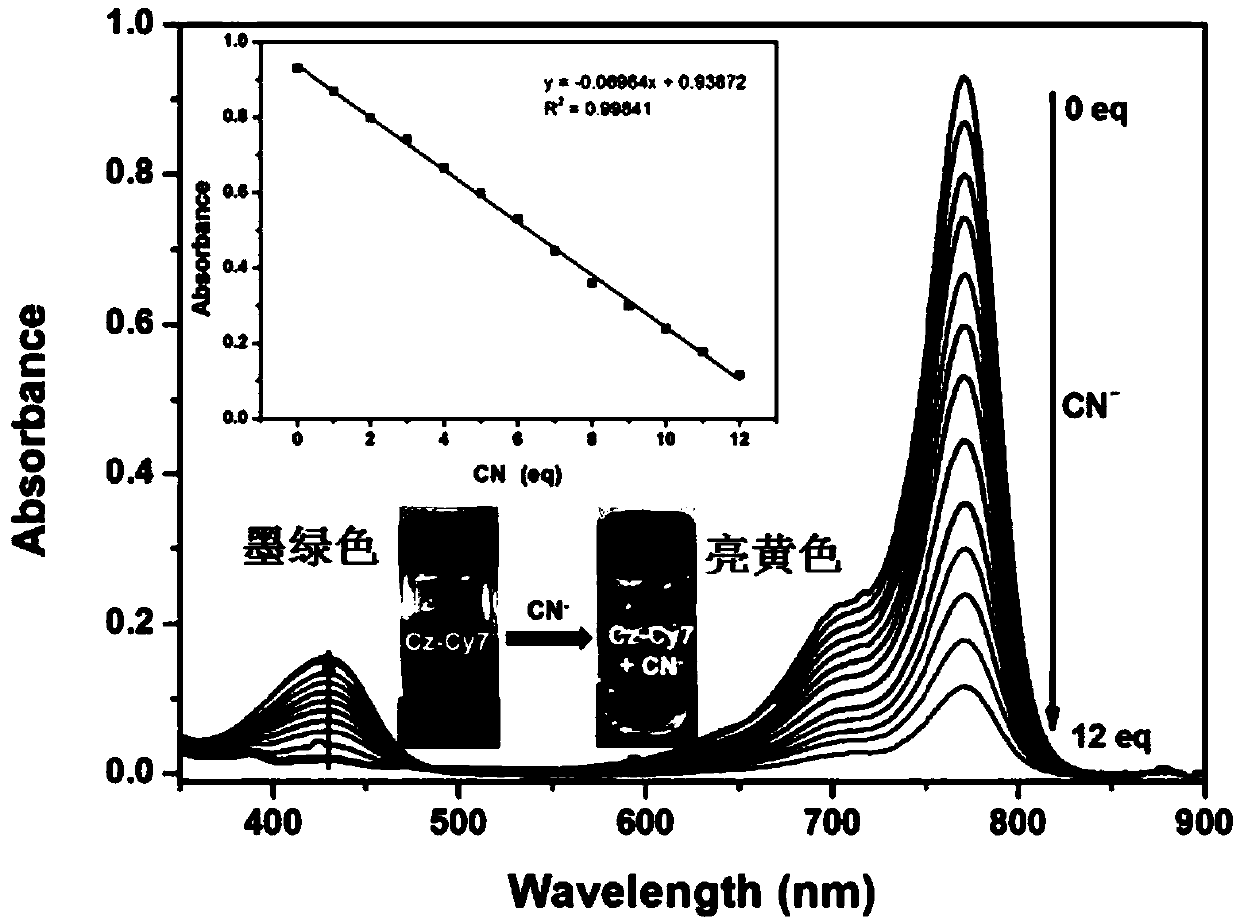
Fluorescent probe detecting cyanide ions and hypochlorous acid and preparation and application thereof
ActiveCN109608382AQuick responseQuick checkOrganic chemistryMaterial analysis by observing effect on chemical indicatorFluoProbesEthyl group
The invention discloses a fluorescent probe detecting cyanide ions and hypochlorous acid and preparation and application thereof. The probe takes p-hydrazinobenzenesulfonic acid and methyl isopropyl ketone as raw materials, and N-ethyl-2,3,3-trimethylindole-5-potassium sulfamate is synthesized through a Fischer reaction and the like; a condensing agent is synthesized through a Vilsmeier-Haack reaction; the condensing agent and N-ethyl-2,3,3-trimethylindole-5-potassium sulfamate are subjected to Knoevenagel Condensation reaction and then subjected to a Suzuki coupled reaction with N-phenyl-3-carbazole boric acid, and the probe is obtained. The probe is a near-infrared probe, and the probe has the advantages of being high in selection, high in sensitivity, rapid in response and the like forthe cyanide ions and hypochlorous acid.
Owner:XIANGTAN UNIV
Preparation method for electronic product plastic shell material
The invention discloses a preparation method for an electronic product plastic shell material. The preparation method comprises the following steps: after 128 parts of PP (propene polymer), 20 parts of oil-filled TPE (thermoplastic elastomer), 12 parts of white oil, 36 parts of polystyrene and 40 parts of triethanolamine are added into a reaction kettle according to parts by weight, heating to 230-235DEG C under the protection of nitrogen, and stirring for 3h; adding 17 parts of dicumyl peroxide and 24 parts of methyl isopropyl ketone, and stirring for 4h under the condition of constant temperature; then, adding reaction resultant into a parallel double-screw granulator; at the temperature of 140-220 DEG C, plasticizing, extruding and smashing to obtain particles; extruding and shaping the particles; putting the particles into a water tank to be cooled; and drying at the temperature of 80 DEG C. The material disclosed by the invention has excellent thermoplastic body mechanical property and machining performance, and the production cost is lowered; the material is nontoxic and pollution-free, and is suitable for mass production; and the material can be recovered after being used, and can be used as a production raw material for injection molding after being cleaned and smashed.
Owner:潘雪峰
Efficient herbicide
InactiveCN106342852AImprove securityEfficient weedingBiocideAnimal repellantsBiotechnologyBensulfuron methyl
The invention discloses an efficient herbicide, made from, by weight, 6-10 parts of sodium polyacrylate, 10-12 parts of chlorimuron-methyl, 6-8 parts of tribenuron-methyl, 6-8 parts of bensulfuron methyl, 40-50 parts of fluoroglycofen-ethyl, 8-12 parts of fluroxypyr, 15-25 parts of chipton, 15-25 parts of mint, 20-30 parts of bensulfuron methyl, 20-30 parts of methyl isopropyl ketone, 6-10 parts of cyhalofop-butyl, 5-7 parts of mefenacet, 20-30 parts of sodium nitrate, 10-12 parts of citric acid, 8-10 parts of sodium hexametaphosphate, and 40-60 parts of water; the efficient herbicide is high in activity, has long acting time and can well inhibit the growth of rice weeds.
Owner:青岛悦邦农种业有限公司
Preparation method of safinamide mesilate A1 crystal form
The invention relates to a preparation method of safinamide mesilate A1 crystal form. The preparation method comprises following steps: methane sulfonic acid is reacted with safinamide at a certain temperature, cooling is carried out, and an obtained reaction liquid is subjected to crystallization at 10 to 30 DEG C, wherein one ingredient selected from acetone, butanone, methyl isobutyl ketone, methyl isopropyl ketone, cyclohexanone, and acetic acid isopropyl ester is taken as a reaction and crystallization solvent, and unexpected crystallization effect is achieved. The maximum yield of the prepared safinamide mesilate A1 crystal form can be higher than 99%, purity is 99.9% or higher in HPLC, impurity content is less than 0.01%, and optical isomer content is less than 0.05%.
Owner:ZHEJIANG MENOVO PHARMA
Method for continuously synthesizing methyl isopropyl ketone
ActiveCN112159317APrecise control of input ratioInput ratio controlOrganic compound preparationCarbonyl compound preparationIsopropylEngineering
The invention provides a method for continuously synthesizing methyl isopropyl ketone. The methyl isopropyl ketone is continuously synthesized in n stages of reaction kettles which are sequentially connected in series. According to the method disclosed by the invention, the purity of the prepared methyl isopropyl ketone reaches 99% or above, and the yield is 73-78%. According to the method, continuous industrial production of methyl isopropyl ketone can be achieved, manual participation operation is reduced, the operation cost is reduced, the raw material input proportion, the raw material input amount and the reaction time can be accurately controlled, automatic control can be achieved, and the industrial production process is safer, more environmentally friendly and more reliable.
Owner:山东智永化工产业技术研究院有限公司
Herbicide
InactiveCN104381301AEnhanced inhibitory effectBroad spectrum herbicideBiocideAnimal repellantsBensulfuron methylHigh activity
Owner:蒋君军
Industrial process for preparation of Clopidogrel hydrogen sulphate
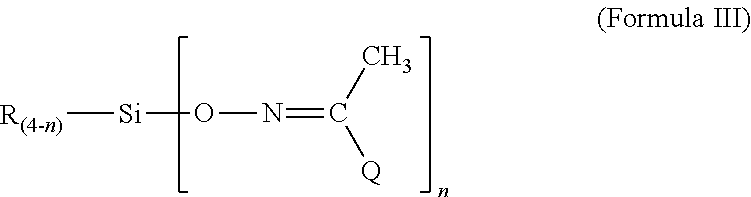
An improved process for the manufacture of Clopidogrel starting from 2-(2-thienyl) ethylamine, which eliminates the isolation of an unstable intermediate like 2-(2-thienyl) ethyl formimine by subjecting it to a one pot cyclization to get 4, 5, 6, 7-tetrahydrothieno (3,2-c) pyridine of Formula II and further reacting with halo-compound of Formula III (where X is Cl or Br) at 20 to 90° C. temperature characterized in a solvent like water and / or dichloroethane in presence of organic or inorganic bases is disclosed herein. This invention further discloses a process for resolution of racemic Clopidogrel into its optical antipodes and converting the dextroclopidogrel base into its known polymorphs namely ‘Form I’ or ‘Form II’ in solvents selected from methyl propyl ketone, methyl isopropyl ketone, diethyl ketone or their mixture thereof, mixture of ethyl acetate and methyl propyl ketone, mixture of ethyl acetate and methyl isopropyl ketone, or mixture of ethyl acetate and diethyl ketone or ethyl acetate.
Owner:IPCA LAB LTD
Preparation method of underwater LED lamp pouring sealant
The invention discloses a preparation method of an underwater LED lamp pouring sealant. The method includes: firstly carrying out stiring hydrolysis condensation polymerization on multifunctional epoxy alkyl alkoxy silane, alkoxy silane, methyl isopropyl ketone and triethylamine, then performing pressure reduced distillation to obtain a component A; stirring phenyl trimethyloxysilane and trifluoromethanesulfonic acid at room temperature to obtain a methyl phenyl siloxane oligomer containing an Si-OH group, and mixing the oligomer with an Al catalyst and an azo compound to obtain a component B; and finally mixing the component A with the component B according to a weight ratio of 3:1-5:1 and stirring them evenly, carrying out decompression and bubble removal, and performing curing, thus obtaining the underwater LED lamp pouring sealant. The sealant for LED lamp packaging obtained by the method provided by the invention has good water proofness and high light transmittance, good adhesive power, toughness and plasticity, and can effectively improve the service life of LED lamps.
Owner:GOLOHO TECH CHANGZHOU CO LTD
Industrial process for preparation of clopidogrel hydrogen sulphate
InactiveUS20080097101A1Easy to operateReadily and commercially availableOrganic chemistryClopidogrel hydrogen sulphateAcetic acid
An improved process for the manufacture of Clopidogrel starting from 2-(2-thienyl) ethylamine, which eliminates the isolation of an unstable intermediate like 2-(2-thienyl) ethyl formimine by subjecting it to a one pot cyclization to get 4, 5, 6, 7-tetrahydrothieno (3,2-c) pyridine of Formula II and further reacting with halo-compound of Formula III (where X is Cl or Br) at 20 to 90° C. temperature characterized in a solvent like water and / or dichloroethane in presence of organic or inorganic bases is disclosed herein. This invention further discloses a process for resolution of racemic Clopidogrel into its optical antipodes and converting the dextroclopidogrel base into its known polymorphs namely ‘Form I’ or ‘Form II’ in solvents selected from methyl propyl ketone, methyl isopropyl ketone, diethyl ketone or their mixture thereof, mixture of ethyl acetate and methyl propyl ketone, mixture of ethyl acetate and methyl isopropyl ketone, or mixture of ethyl acetate and diethyl ketone or ethyl acetate.
Owner:IPCA LAB LTD
Herbicide containing tralkoxydim and preparation method thereof
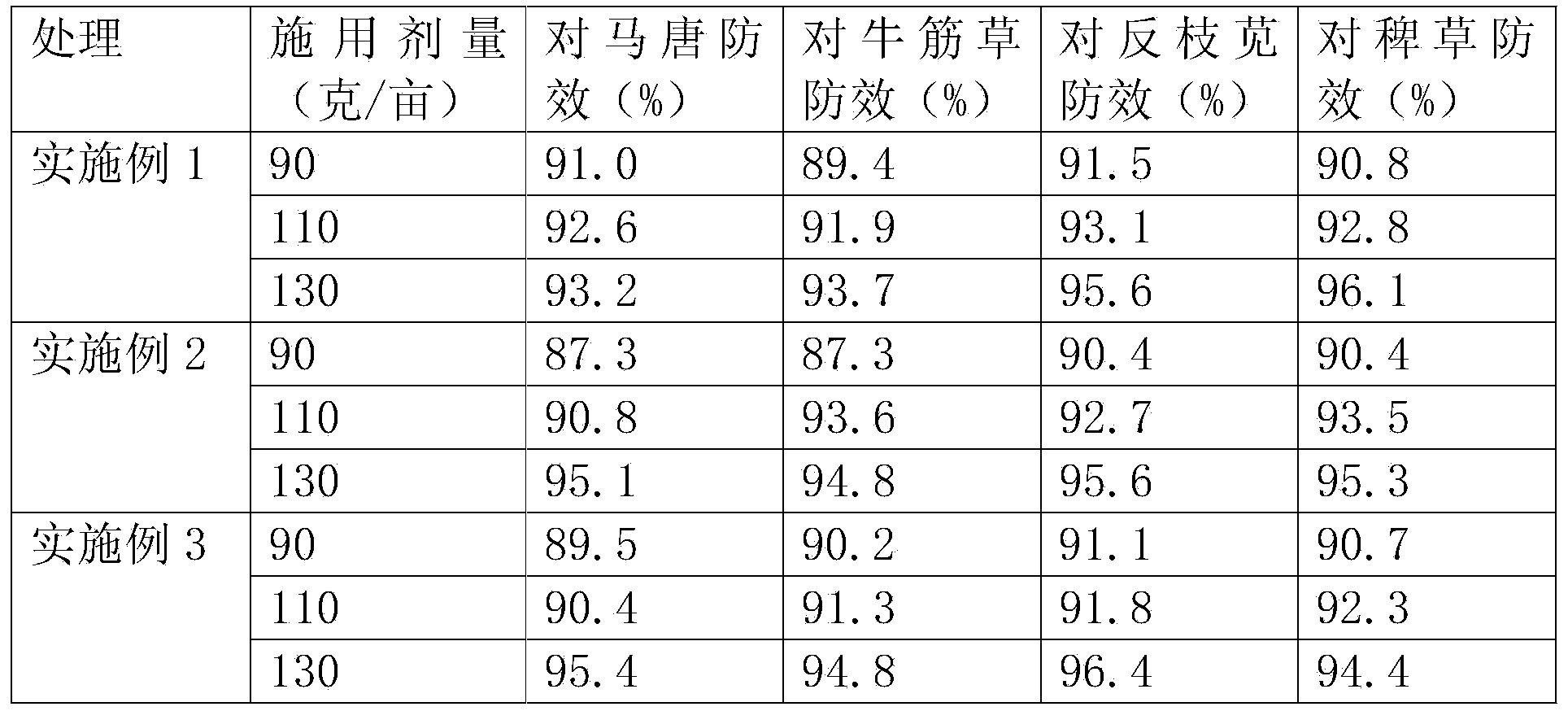
The invention discloses an herbicide containing tralkoxydim and a preparation method thereof. The herbicide comprises the following components (by weight): 10-20 parts of tralkoxydim, 15-30 parts of methyl 2-amino-3-chlorobenzoate, 5-15 parts of bensulfuron-methyl, 8-12 parts of 2,3-difluoro-5-chloropyridine, 10-20 parts of 2-chloro-4-diethylamine-6-isopropylamine-1,3,5-triazine, 10-14 parts of acetochlor emulsifiable concentrates, 10-20 parts of atrazine, 1-3 parts of an organic silicon defoamer, 5-15 parts of mefenacet, 20-30 parts of sodium hexametaphosphate, 5-15 parts of sodium nitrate, 10-20 parts of citric acid, 20-30 parts of methyl isopropyl ketone, 5-15 parts of cyhalofop-butyl, 7-10 parts of bispyribac-sodium, 5-15 parts of bentazone, and 1-3 parts of a pesticide adjuvant. The prepared herbicide has the advantages of high efficiency, low toxicity, low residue, low cost and good control effect on grassy and broadleaf weeds.
Owner:NANJING PHARMATECHS
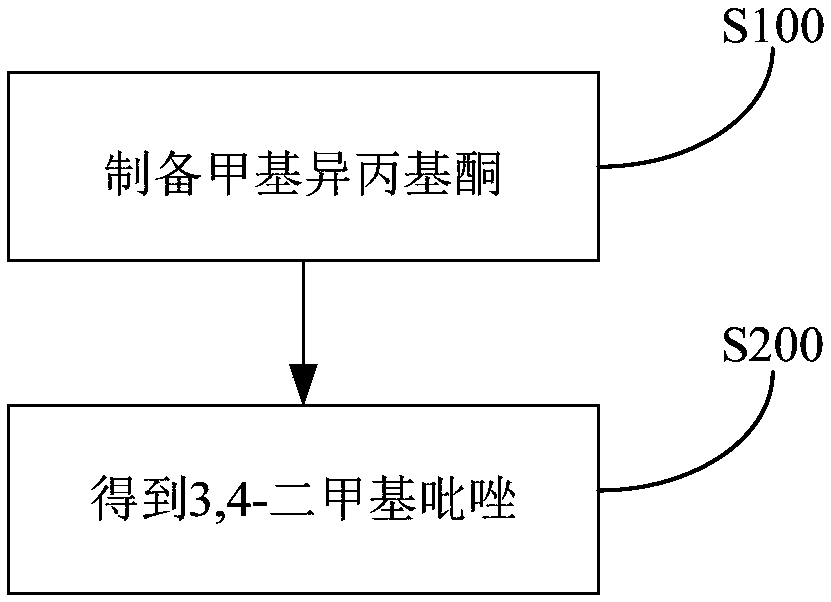
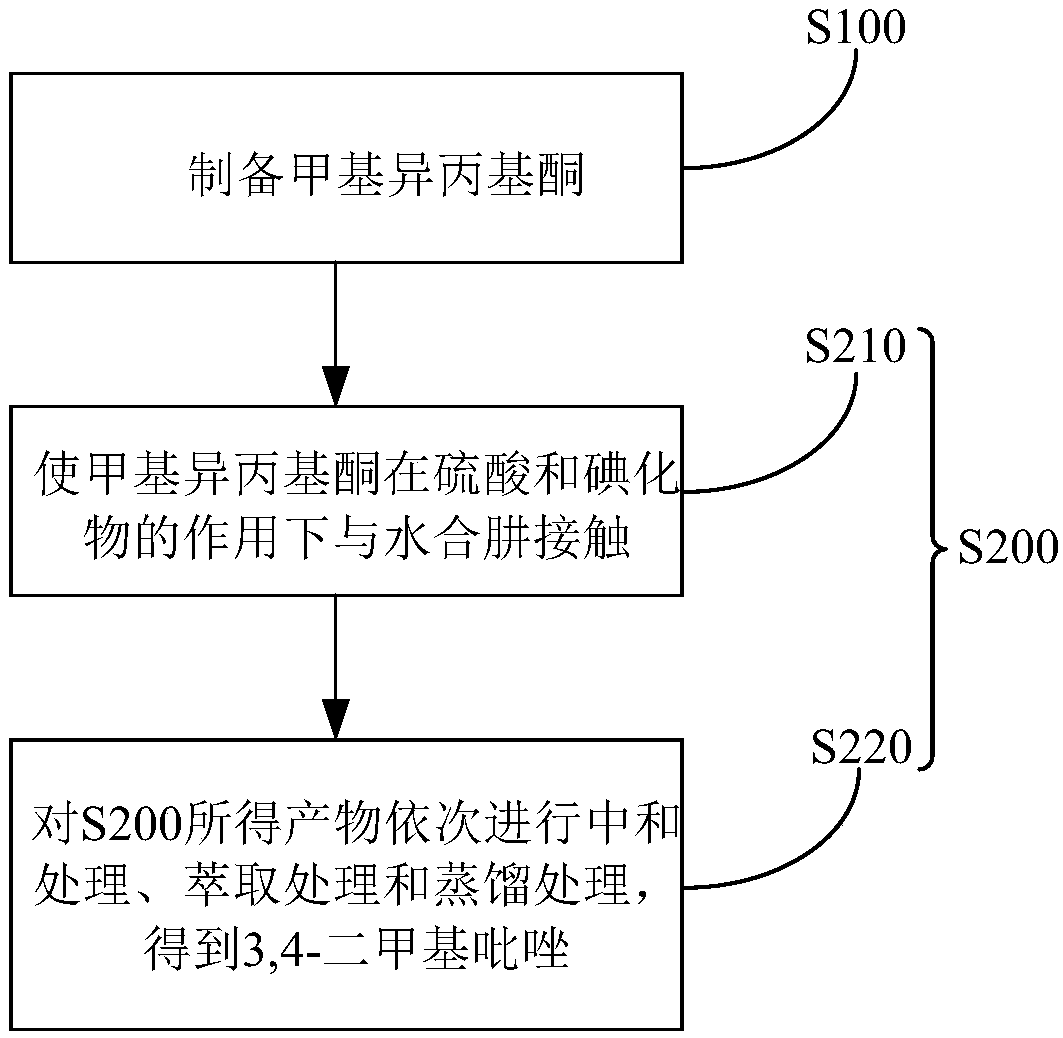
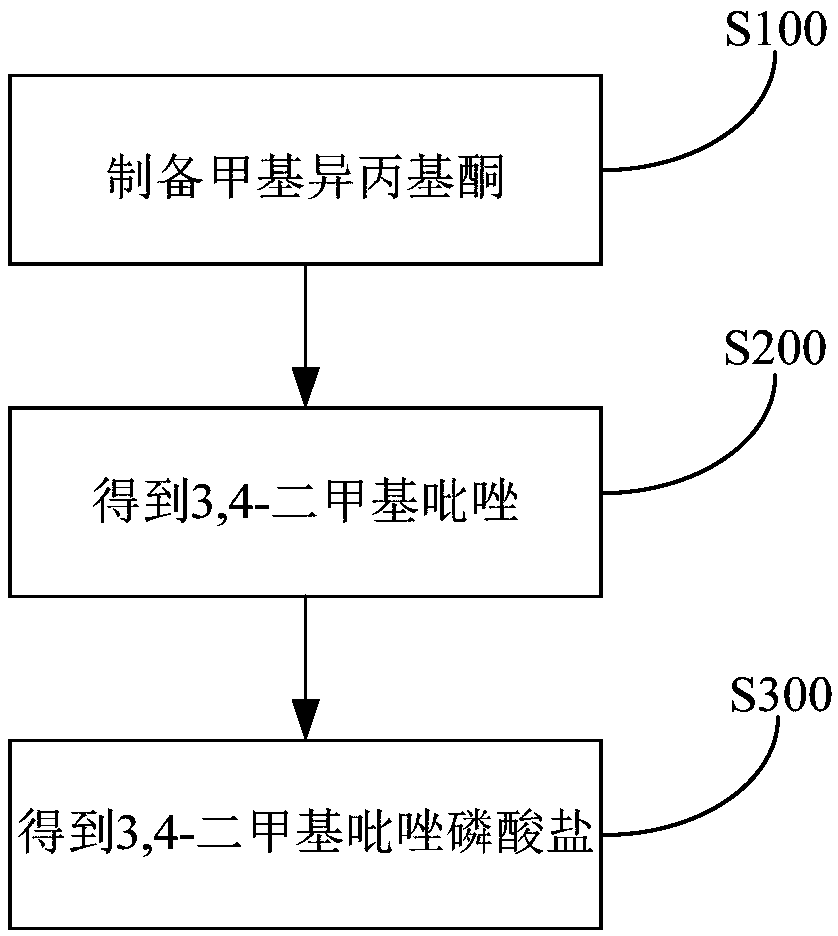
Method for preparing 3,4-dimethylpyrazole and phosphate and metal organic complex thereof
InactiveCN109651252ALow costLower requirementGroup 2/12 organic compounds without C-metal linkagesZinc organic compoundsIodidePhosphate
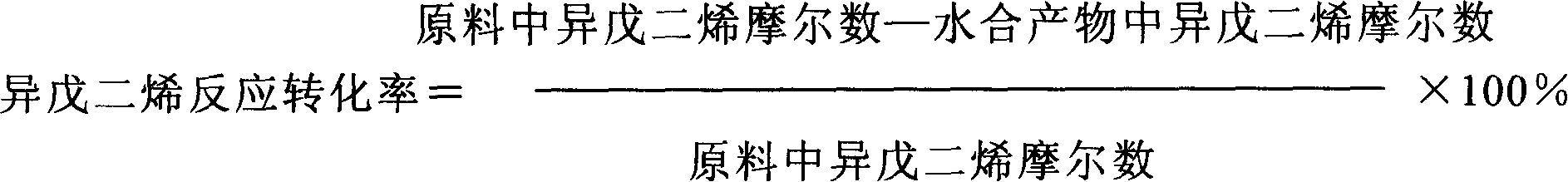
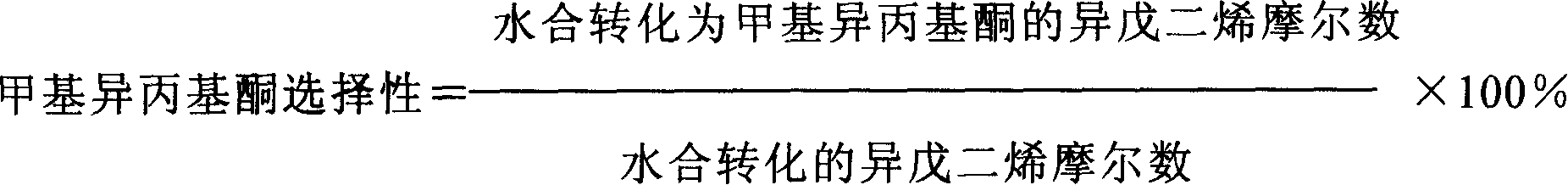
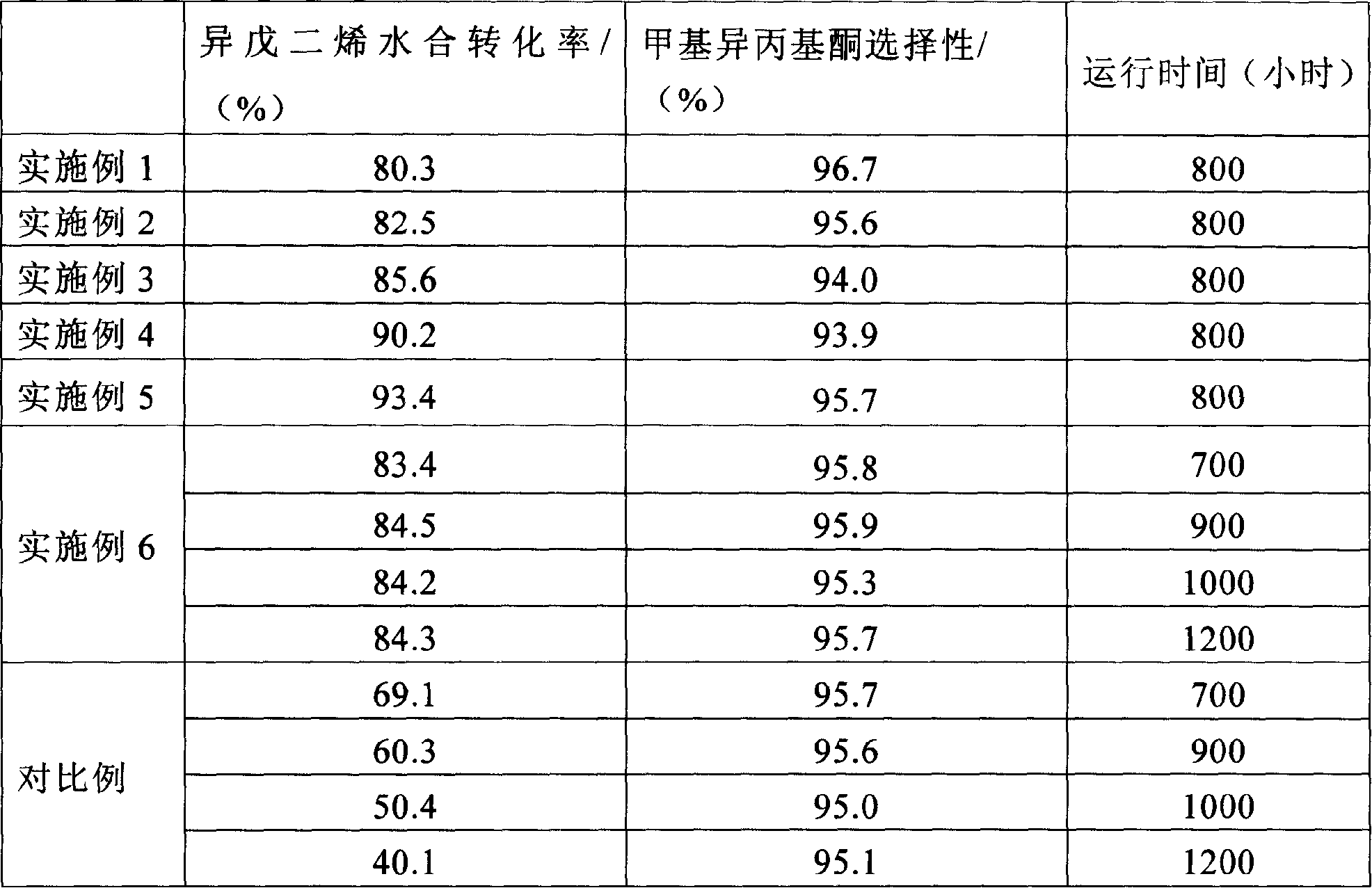
The invention discloses a method for preparing 3,4-dimethylpyrazole and phosphate and metal organic complex thereof. The method for preparing the 3,4-dimethylpyrazole comprises the steps that (1) isoprene is in contact with water under the action of a catalyst to obtain methyl isopropyl ketone; (2) the methyl isopropyl ketone is in contact with hydrazine hydrate under the action of sulfuric acid and iodide to obtain the 3,4-dimethylpyrazole. The method for preparing the 3,4-dimethylpyrazole has the advantages that the isoprene is taken as a raw material, the preparation method is simple, and the yield and purity of the product are high.
Owner:SINOCHEM AGRI LINYI R&D CENT CO LTD
Efficient and environmentally-friendly paint diluent and preparation method thereof
The invention relates to an efficient and environmentally-friendly paint diluent and a preparation method thereof. The paint diluent comprises ethyl acetate, butyl acetate, ethanol, acetone, dichloromethane, dichloroethane, ethylene glycol monoethyl ether acetate, cyclohexanone, tetrabutyl orthosilicate, an ethyl cellosolve, methyl isopropyl ketone, turpentine and an aromatic. The preparation method comprises the following steps: mixing the ethyl acetate, butyl acetate, ethanol, acetone, dichloromethane, dichloroethane, ethylene glycol monoethyl ether acetate, cyclohexanone, methyl isopropyl ketone and turpentine, adding an acid-base regulator to regulate the pH value to 5, performing stirring at 50-60 DEG C for 1-2 h, adding the tetrabutyl orthosilicate, the ethyl cellosolve and the aromatic, performing sufficient stirring, and cooling the obtained mixture to room temperature. The efficient and environmentally-friendly paint diluent has the advantages of good leveling property after being sprayed, no scaling, freeness of substances harmful to human bodies, and effectiveness in improvement of the smell generated after paint spraying by adding aromatic hydrocarbon for improving thesmell.
Owner:ANQING XINXIANGRUI CHEM CO LTD
Method for preparing polyester containing isosorbide
Owner:CHINA PETROLEUM & CHEM CORP +1
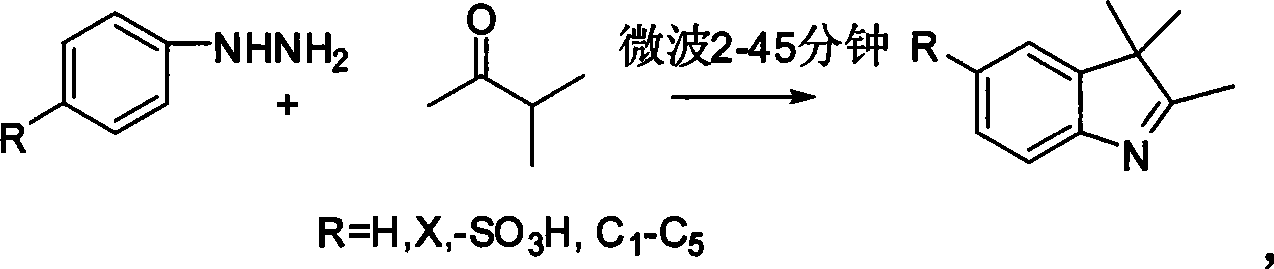
Microwave synthesizing method for 2, 3, 3-trimethyl -3H-indole and its derivatives
InactiveCN101544590AReduce pollutionShort reaction timeOrganic chemistryEnergy based chemical/physical/physico-chemical processesAcetic acidOrganic solvent
A microwave synthesizing method for 2, 3, 3-trimethyl -3H-indole and its derivatives, which indicates dissolving phenyl hydrazine or phenyl hydrazine derivates together with methyl isopropyl ketone with the molar ratio of 1:1-1:20 in acetic acid, putting the solution into a reaction container, irradiating with microwave for 2-45 minutes at 200-850W, finishing the reaction in short minute and severing the solution after concentrated and cooled down with conventional method. This microwave synthesizing method employs microwave heating, and the fire power and time show inverse ratio when microwave acts. Compared to existing technology which needs several hours to more than ten hours, this invention considerably shorten the time of reaction, the acetic acid during the reaction can be recovered and reused and there adds no other organic solvent and catalyse ache which reduces the pollution of the environment.
Owner:JIANGXI SCI & TECH NORMAL UNIV
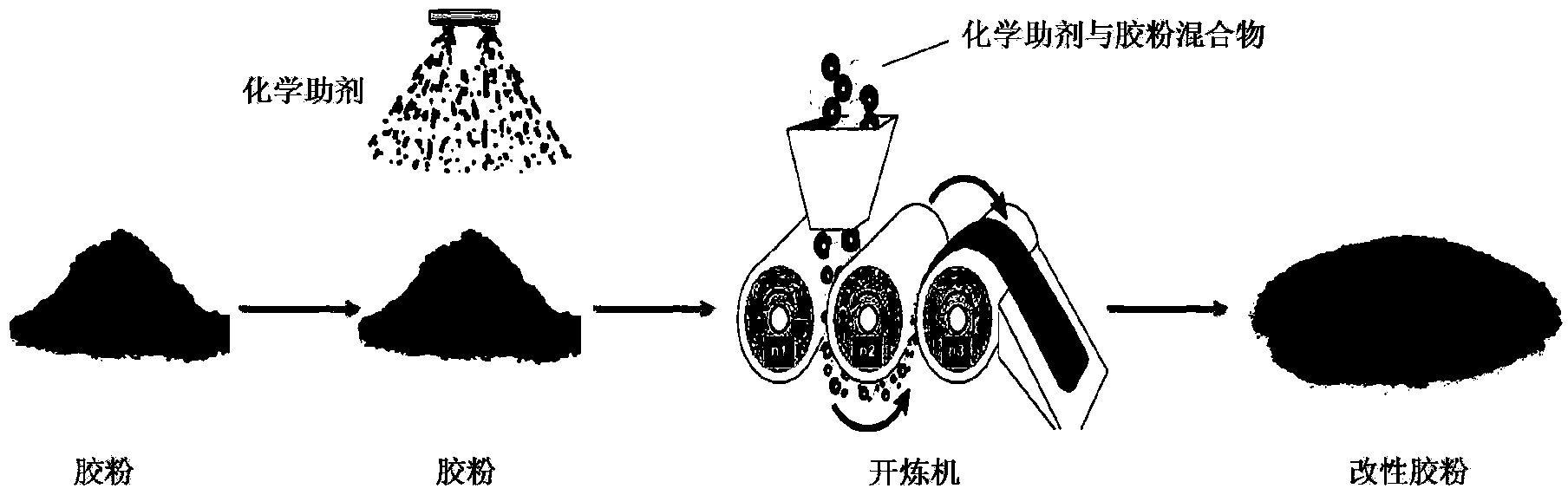
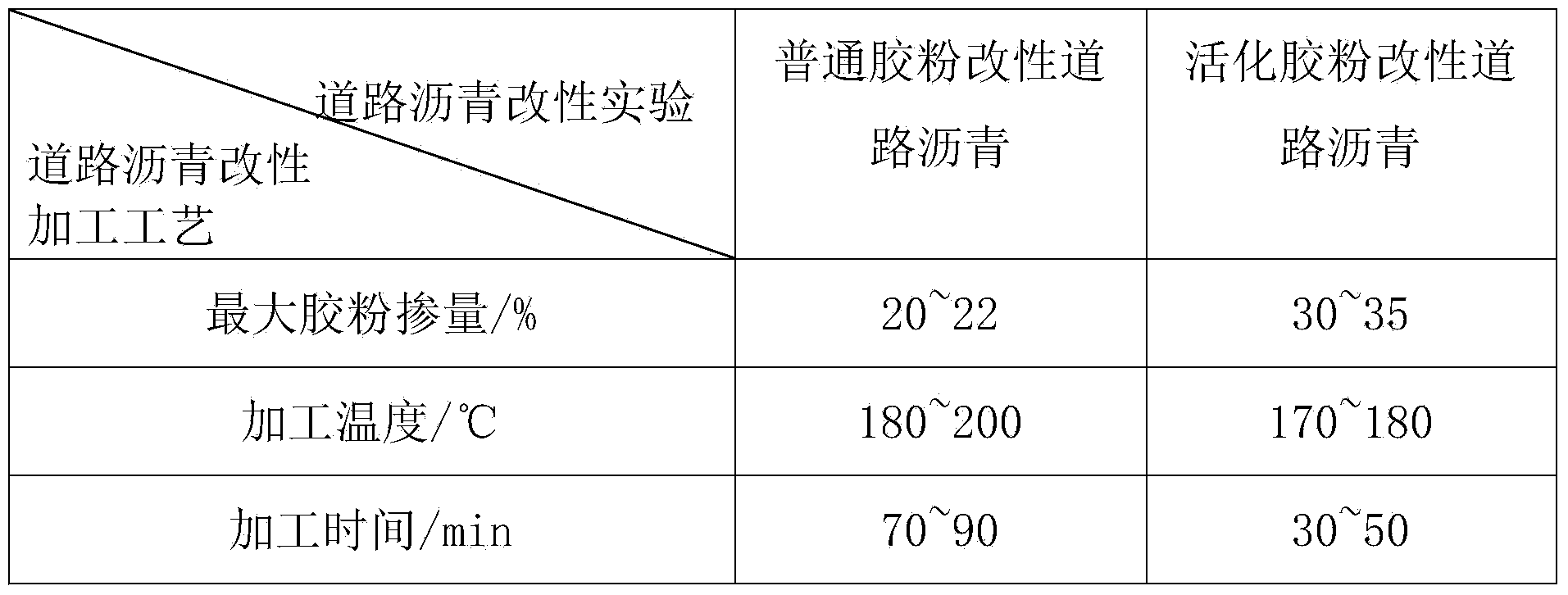
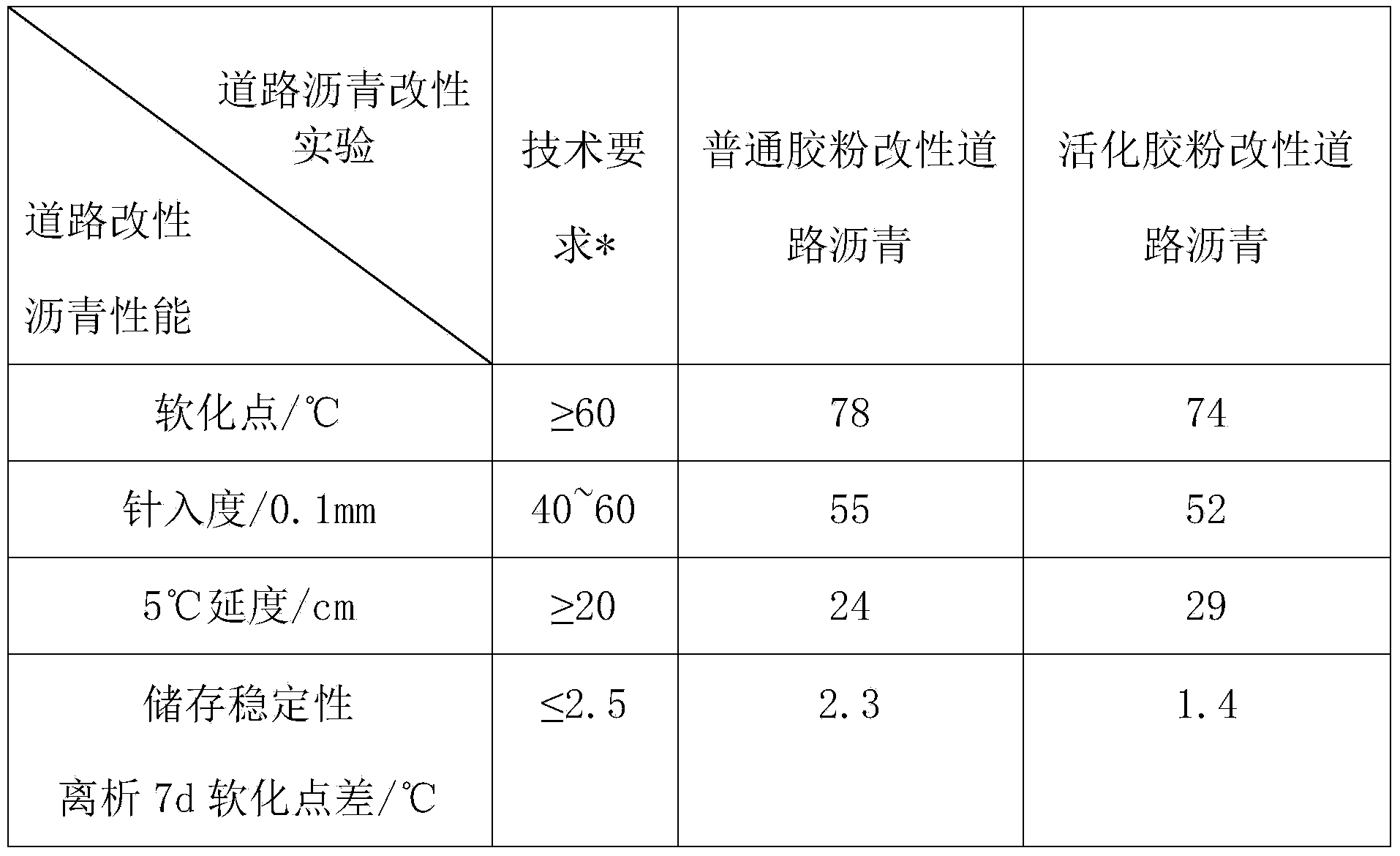
Preparation method and application of asphalt modifier for roads
InactiveCN104177851ARealize activation modificationIncrease dosageBuilding insulationsAging resistanceDimethoxymethane
The invention relates to an asphalt modifier for roads, which is prepared by the following steps: spraying a quantitative chemical assistant onto the surface of rubber powder, and repeatedly rolling by an open mill. The chemical assistant is composed of at least one of 1-diethoxy propane, 1,1-dimethoxymethane, 2,2-dimethoxypropane, isooctane, methyl isopropyl ketone, methyl tetrahydrofuran, petroleum ether and trichloroacetic acid. The weight ratio of the chemical assistant to the rubber powder is (5-20):(80-95). The asphalt modifier has higher compatibility with matrix asphalt, improves the high / low temperature properties, storage stability and aging resistance of the road modified asphalt, comprehensively improves the road usage properties of the asphalt, and thus, can be used for preparing high-grade road modified asphalt.
Owner:BEIJING ORIENTAL YUHONG WATERPROOF TECH CO LTD
Special paint for plastic cement material
The invention discloses a special paint for plastic cement materials, which is characterized by comprising a component A and a component B, and is matched with a thinner, wherein the component A comprises the following ingredients in percentage by weight: 48-52% of acrylic acid resin, 23-27% of polyester resin, 14-16% of epoxy modified resin, 0.5-1% of accessory ingredient and 6-10% of butyl acetate; the component B is a curing agent; the thinner comprises the following ingredients in percentage by weight: 90% of methyl isopropyl ketone (MIBK) and 10% of butyl acetate; the ratio of the component A to the component B to the thinner is 4:1:2; and the component A and the component B are mixed and evenly stirred in ratio. The special paint disclosed by the invention has excellent adhesive force and good flexibility and glossiness and is convenient to construct.
Owner:HANGZHOU LIWEI CHEM INDAL PAINT
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

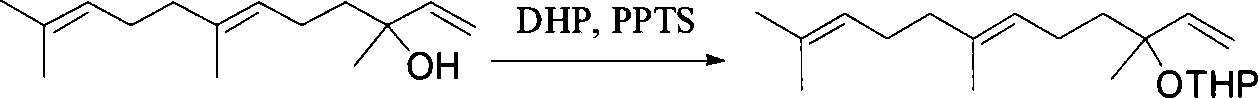
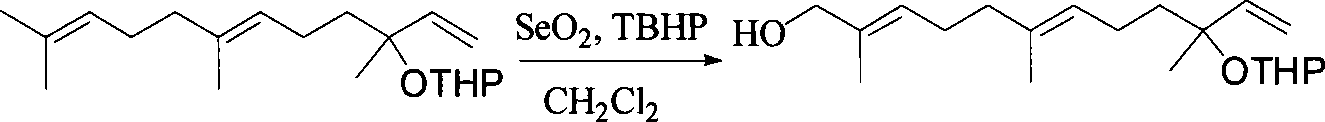
![Synthesis method of 2-[2-(4-fluorophenyl)-2-oxa-1-phenylethyl]-4-methyl-3-oxa-N-phenylpentanamide Synthesis method of 2-[2-(4-fluorophenyl)-2-oxa-1-phenylethyl]-4-methyl-3-oxa-N-phenylpentanamide](https://images-eureka.patsnap.com/patent_img/3b82a23d-51dc-4945-890c-a169aa31ba8f/2011103568037100002DEST_PATH_IMAGE001.PNG)