Treatment of joint conditions
a joint condition and treatment method technology, applied in the field of joint condition treatment, can solve the problem of inflammation of joint conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0051]A randomized, placebo-controlled, double-blind study was performed to evaluate the efficacy and safety of two doses of intra-articular (IA) injection of Ampion™ in adults with pain due to osteoarthritis of the knee (OAK).
Primary Objective
[0052]To evaluate whether the efficacy of 10 mL Ampion™ versus 10 mL placebo is greater than the efficacy of 4 mL Ampion™ versus 4 mL placebo IA injection in improving knee pain, when administered to patients suffering from OAK.
Study Subjects
[0053]Study subjects were male and female adult patients who were 40 years to 85 years old (inclusive). Eligible patients were ambulatory but suffering from moderate to moderately severe pain from OAK in the index knee, as evidenced by a rating of at least 1.5 on the WOMAC Index 3.1, five-point Likert Pain Subscale at screening. Patients must have had an index knee that was symptomatic for greater than six months with a clinical diagnosis of OAK, which was supported by radiological evidence (Kellgren-Lawre...
example 2
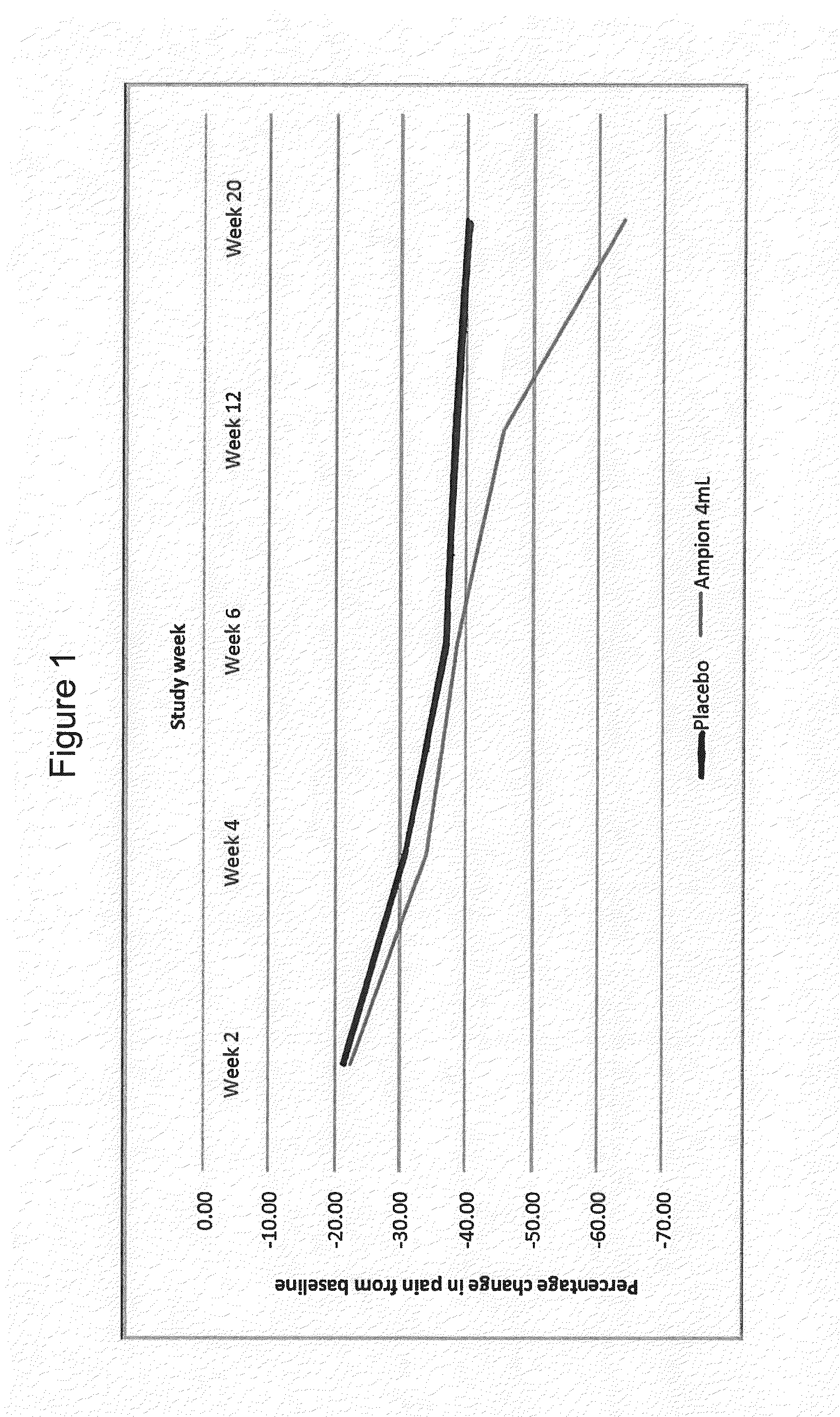
[0072]A prospective Phase I / II study was performed to evaluate the safety and efficacy of three intra-articular injections of Ampion™ (4 mL) administered two weeks apart in adults with pain due to osteoarthritis of the knee.
Primary Objectives
[0073]The primary objective of Phase I was to evaluate the safety of Ampion™ 4 mL administered as three IA injections, two weeks apart, in patients suffering from OAK of the knee from baseline to Week 20.
[0074]The primary objective of Phase II was to evaluate the safety and efficacy of Ampion™ 4 mL versus placebo injection from baseline to Week 20, when administered as 3 IA injections, in improving knee pain in patients suffering from OAK of the knee.
Study Subjects
[0075]For both the Phase I and Phase II studies, subjects were male and female adult patients who were 40 years to 85 years old (inclusive) with OAK knee pain. Eligible patients were required to be ambulatory and the index knee must have been symptomatic for greater than six months wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com