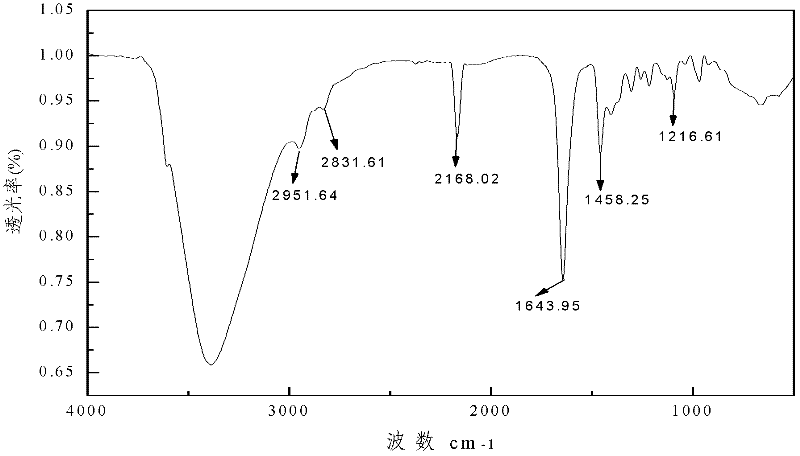
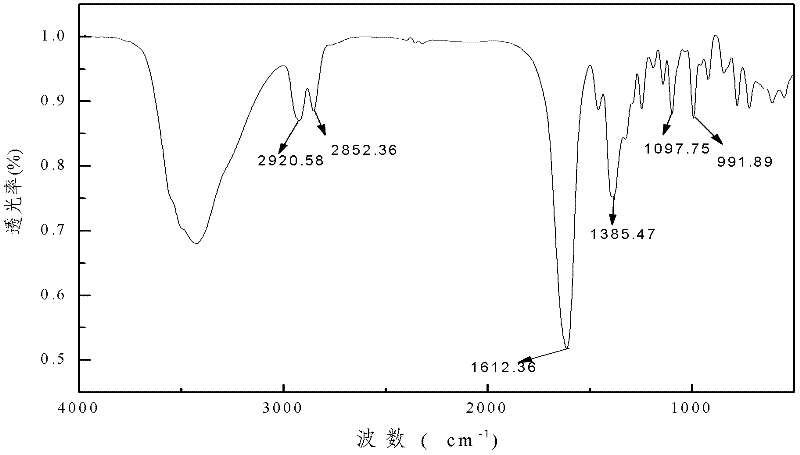
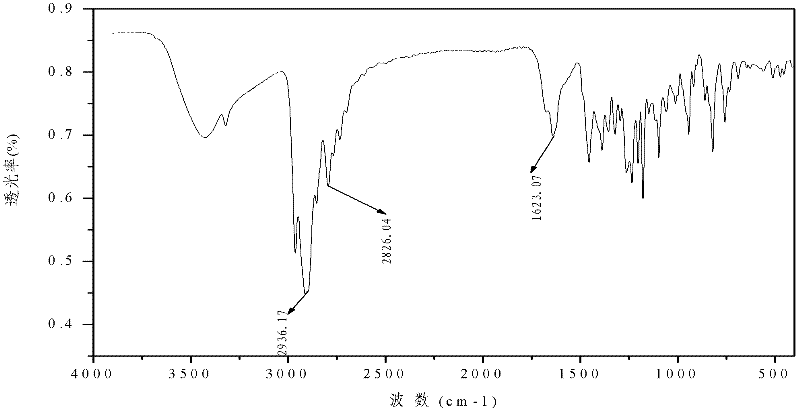
Metal complex of novel double piperidine derivative with symmetric structure
A metal complex, symmetrical structure technology, applied in the direction of organic compound/hydride/coordination complex catalyst, copper organic compound, zinc organic compound, etc., can solve the synthesis method of metal complex without bipiperidine derivatives, etc. problem, to achieve the effect of good catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Embodiment 1: the synthesis of bispiperidine ligand 1b
[0040] The structural formula of bispiperidine ligand 1b is:
[0041]
[0042] N 2 Under protection, add 1g (7.94mmol) of bispiperidine and 15ml of acetone into the reaction flask, stir until it dissolves, and cool down to 0°C, add 4.4g (32mmol) of anhydrous potassium carbonate as an ancidic agent, and then slowly add chlorine 2ml (32mmol) of acetonitrile was dropped within 0.5h, and the reaction was carried out at room temperature, and the progress of the reaction was detected by TLC (color development with iodine). After detecting that no bispiperidine remains, remove potassium carbonate by filtration, concentrate the filtrate, and recrystallize the residue with ethyl acetate to obtain bispiperidine ligand 1b as a white solid, 1.2 g, melting point: 130.9-132.0°C, yield 74% . 1 HNMR (CDCl 3 , 400MHz) δ: 1.60 (s, 2H, CH 2 bridge), 2.09(s, 2H, CH), 2.70~2.74(m, 4H, cycl.NCH 2ax ), 2.85~2.88 (m, 4H, cycl.NC...
Embodiment 2
[0043] Embodiment 2: the synthesis of bispiperidine ligand 2b
[0044] The structural formula of bispiperidine ligand 2b is:
[0045]
[0046] N 2Under protection, add 40.8g (6.7mmol) of bispiperidine, 15ml of acetone, and 2.78g (20.1mmol) of anhydrous potassium carbonate into the reaction flask as an acid application agent, drop to 0°C, and slowly add 2.16ml of ethyl chloroacetate (20.1mmol), dropwise finished within 0.5h, raised to room temperature, TLC detection of reaction progress (iodine color). After detecting that there is no remaining bispiperidine, remove potassium carbonate by filtration, dry the filtrate with molecular sieves, concentrate to remove the solvent and excess ethyl chloroacetate, and add 10ml of methanol to the obtained yellow sticky substance, stir until it dissolves, and then add 40% NaOH solution 4ml, heated to reflux. TLC detection of reaction progress (iodine color development), after detection of no intermediate remaining, lower to room temp...
Embodiment 3
[0047] Embodiment 3: the synthesis of bispiperidine ligand 3b
[0048] The structural formula of bispiperidine ligand 3b is:
[0049]
[0050] Under nitrogen protection and ice-water bath cooling, add (R)-mandelic acid 3.07g (20.2mmol) and N-hydroxysuccinimide 2.32g (20.2mmol) successively in a three-necked flask equipped with 50mL THF (tetrahydrofuran), and react After the substance was completely dissolved, 3.87 g (20.2 mmol) of EDCI was added, stirred for 30 min, raised to room temperature for reaction, and TLC was used to detect the reaction progress. After detecting that there is no remaining (R)-mandelic acid, add 0.8 g (6.7 mmol) of bispiperidine dropwise to the above reaction solution under cooling in an ice-water bath, and complete the dropping within 0.5 h, stir for 30 min, rise to room temperature for reaction, and detect by TLC reaction process. After detecting that there is no remaining bispiperidine, wash with saturated sodium chloride, dry over anhydrous so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com