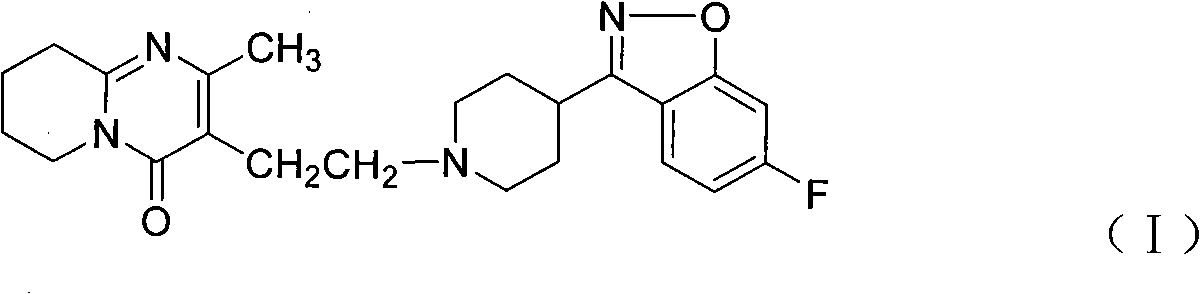
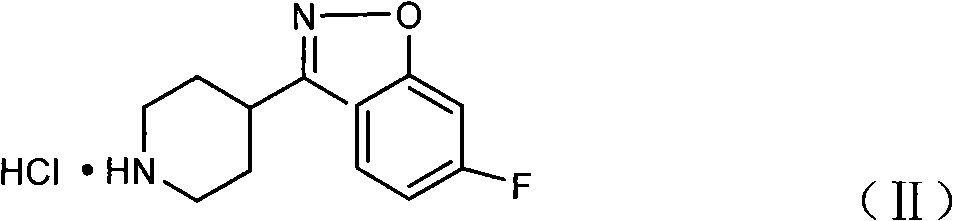
Method for preparing 6-fluoro-3-(4- piperidyl)-1,2-benzo isoxazole hydrochlorate
A technology of benzisoxazole hydrochloride and piperidinyl, which is applied in the field of chemical synthesis and can solve problems such as low water solubility, difficult separation, and affecting the yield of risperidone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Under stirring at room temperature, mix 13.6 g (0.049 mol) of 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride, 6.9 g (0.123 mol) of solid KOH powder and 109 ml of acetone, and heat up to 55 ~60°C, reflux reaction for 2 hours; after the reaction, add an appropriate amount of anhydrous sodium sulfate to dry, cool to room temperature, stir for 30 minutes, and filter; pass HCl gas into the filtrate, and crystallize; filter and dry to obtain 6-fluoro - 3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride 12.25g, yield 97.47%, content>99%.
Embodiment 2
[0029] Under stirring at room temperature, 13.6 g (0.049 mol) of 2,4-difluorophenyl (4-piperidinyl) ketone oxime hydrochloride, 8.23 g (0.147 mol) of solid KOH powder and N, N-dimethyl Mix 136ml of formamide, heat up to 40-45°C, and reflux for 3 hours; after the reaction, add an appropriate amount of anhydrous sodium sulfate, cool down to room temperature, stir for 30 minutes, and filter; pass HCl gas into the filtrate, and crystallize; filter , and dried to obtain 11.69 g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, with a yield of 93.01% and a content of >99%.
Embodiment 3
[0031] Under stirring at room temperature, mix 24 g (0.1 mol) of 2,4-difluorophenyl (4-piperidinyl) ketone oxime, 11.2 g (0.2 mol) of solid KOH powder and 120 ml of N, N-dimethylacetamide , heated up to 45-50°C, and refluxed for 1 hour; after the reaction, add an appropriate amount of anhydrous sodium sulfate, cool down to room temperature, stir for 30 minutes, and filter; pass HCl gas into the filtrate, and crystallize; filter, dry, and get 24.55 g of 6-fluoro-3-(4-piperidinyl)-1,2-benzisoxazole hydrochloride, yield 95.7%, content>99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com