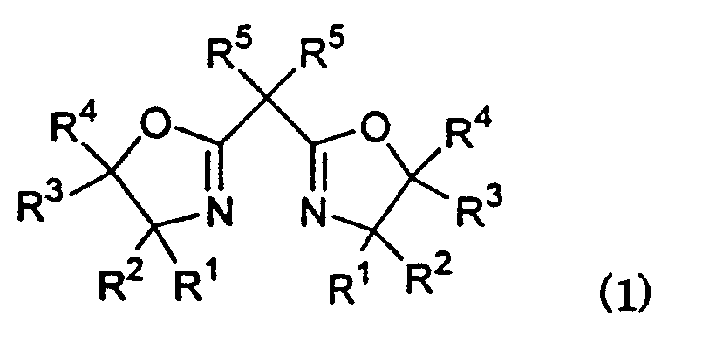
Asymmetric copper compound and cyclopropanation reaction with it
A copper complex and asymmetric technology, applied in the field of asymmetric copper complexes and cyclopropane compounds, can solve the problems of poor stability, difficult handling, complex copper complexes, etc., and achieve the effect of low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1A
[0199] After replacing the atmosphere in a glass Schlenk tube with an inner volume of 50 ml with nitrogen, 9.8 mg (0.022 mmol) of 2,2'-isopropylidenebis{(4R)-4-phenyl-5 was added at room temperature, 5-diethyl-2-oxazoline}, 4mg (0.02mmol) of copper acetate monohydrate and 5ml of ethyl acetate, after stirring the mixture for 0.5 hours, added 3.0mg (0.02mmol) of trifluoromethanesulfonic acid , and the mixture was stirred at room temperature for 1 hour to prepare a complex catalyst solution.
Embodiment 1B
[0201] 11 g (100 mmol) of 2,5-dimethyl-2,4-hexadiene was added to the complex catalyst prepared in Example 1A. Then 10 ml of a solution of ethyl diazoacetate in toluene (containing 10 mmol of ethyl diazoacetate) was added thereto within 2 hours, during which the reaction temperature was kept at 20°C. The mixture was then kept at 20° C. for 30 minutes. The yield and ratio of trans / cis isomers of ethyl chrysanthemum acid as the product were analyzed by GC, and the optical purity was analyzed by LC. The yield of ethyl chrysanthemic acid was 88.4% (relative to the ethyl diazoacetate added), trans / cis = 74 / 26, the optical purity was 84% e.e. (trans) and 24% e.e. (cis ).
Embodiment 1C
[0203] The same reaction as in Example 1B was carried out, except that tert-butyl diazoacetate was used instead of ethyl diazoacetate in Example 1B. After the product was converted to the 1-menthyl derivative, its optical purity was analyzed by GC.
[0204] The yield of tert-butyl chrysanthemic acid was 81.5% (relative to the added tert-butyl diazoacetate), trans / cis = 83 / 17, optical purity was 94% e.e. (trans) and 60% e.e. ( cis).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com