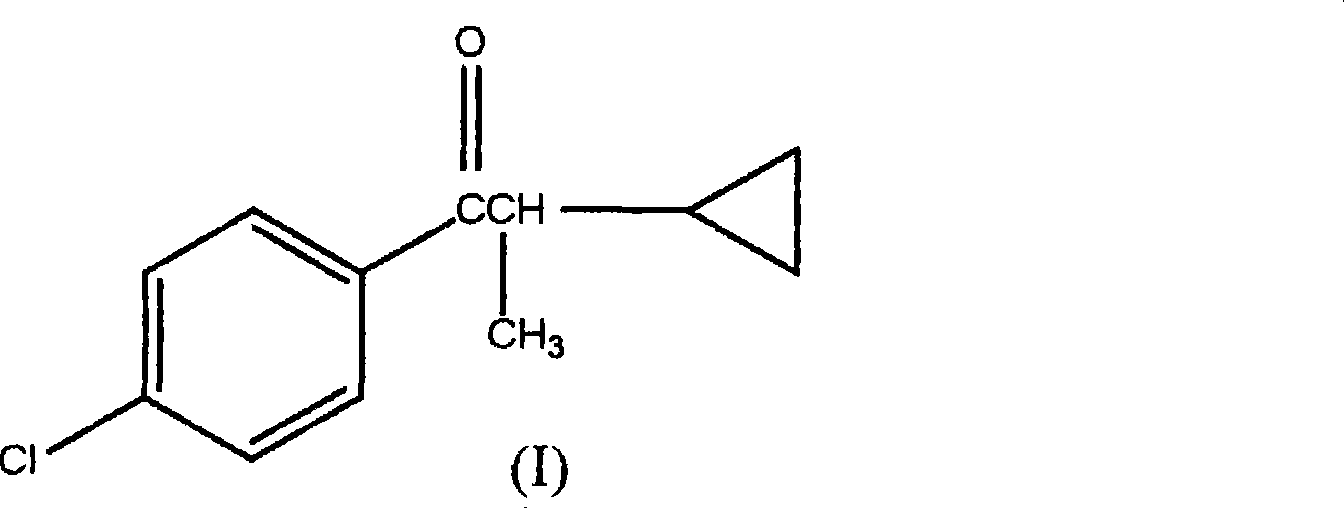
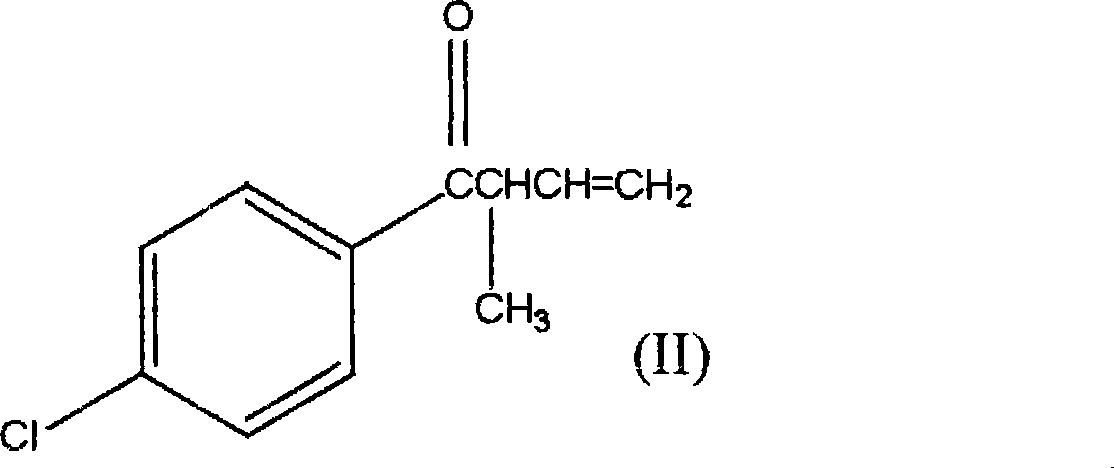
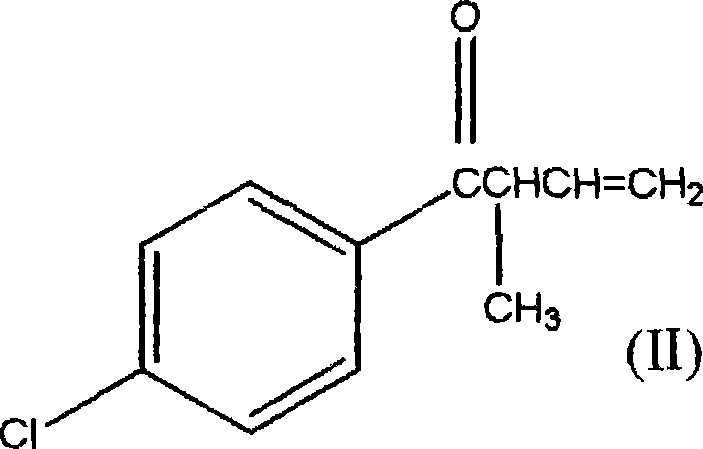
1-(4-chlorophenyl)-2-cyclopropyl-1-acetone and preparation method for intermediate thereof
A technology of chlorophenyl and cyclopropyl, which is applied in the field of preparation of 1--2-cyclopropyl-1-propanone, can solve problems such as difficulty in realizing industrial production, and achieve the effect of simplifying the reaction process and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Synthesis method of 1-(4-chlorophenyl)-2-methyl-3-buten-1-one.
[0038] Add 6.78g (0.05mol) p-chlorobenzonitrile to 45ml tetrahydrofuran solution, and cool 13.0g (0.2mol) zinc powder to 10°C. Then, 2.67g (0.02mol) of aluminum trichloride was added to the reaction mass, and the temperature rose to above 15°C. Then 10.12 g (0.075 mol) of butenyl bromide dissolved in 50 ml of tetrahydrofuran solution were added dropwise within 1.5 hours. After the addition, it was stirred for 3.5 hours at a temperature of 35-40°C. After the reaction was completed, 60ml of n-hexane was added and cooled to 0°C. Slowly add 36ml of 2M hydrochloric acid at the temperature of the reaction mass at 0°C, and stir for 10 minutes.
[0039] The layers were separated, and the organic layer was washed successively with 2M hydrochloric acid solution (2 times 35 ml), 5% sodium bicarbonate solution (2 times 35 ml), and sodium chloride solution (3 times 35 ml). Dry over anhydrous sodium sulfat...
Embodiment 2
[0041] Example 2 Synthesis method of 1-(4-chlorophenyl)-2-methyl-3-buten-1-one.
[0042] According to Example 1 of the present invention without adding aluminum trichloride, 5.94 g of 1-(4-chlorophenyl)-2-methyl-3-buten-1-one was obtained. The yield is 61%, and the content of the product is 81.5% through gas chromatography analysis.
Embodiment 3
[0043] Example 3 Synthesis method of 1-(4-chlorophenyl)-2-methyl-3-buten-1-one.
[0044] Add 27.5g (0.2mol) of p-chlorobenzonitrile and 52.0g (0.8mol) of zinc powder into 180ml of tetrahydrofuran solution, and cool the reaction mass to 10°C. Add 10.68g (0.08mol) of aluminum trichloride to the reaction mass, and the temperature rises above 25°C. Then 27.15 g (0.3 mol) of butenyl chloride dissolved in 153 ml of tetrahydrofuran solution were added dropwise within 1.5 hours. The temperature rises above 30°C. After the addition, it was stirred for 4 hours at a temperature of 35-40°C.
[0045]After the reaction was completed, 210 ml of n-hexane was added and cooled to 0°C. The reaction mass was slowly added with 160ml of 2M hydrochloric acid at a temperature of 0°C. The layers were separated, and the organic layer was washed successively with 2M hydrochloric acid solution (2 times 100 ml), 5% sodium bicarbonate solution (2 times 120 ml), and sodium chloride solution (3 times 100...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com