Method for preparing 6beta,7beta-methylene-steride-3beta,5beta-diol
A methylene and steroid technology, applied in the field of 6β, can solve the problems of high cost of environmental protection, lengthy reaction steps, expensive reaction reagents, etc., and achieve the effect of cheap raw materials, easy preparation, and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
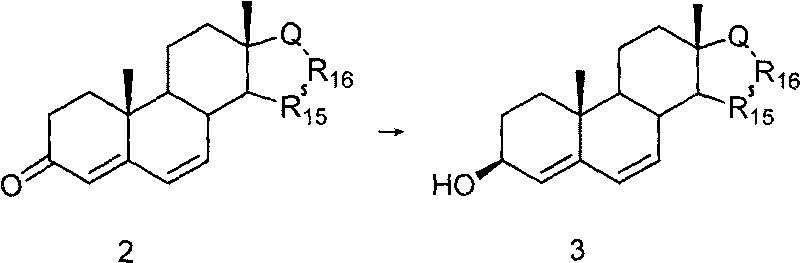
[0028] At room temperature, Δ 4,6 - Add sodium borohydride (537 mg, 14.2 mmol) to a solution of androsdiene-3,17-dione (1.0 g, 3.5 mmol) in absolute ethanol (100 mL), and keep stirring overnight. After the reaction was completed, the solvent was removed by vacuum extraction to obtain a white solid. The white solid was placed in water and stirred for 30 min. It was then extracted with dichloromethane (3 x 100 mL). The organic layer was washed successively with aqueous sodium bicarbonate solution and water, dried over anhydrous magnesium sulfate, filtered, concentrated, recrystallized from ethyl acetate / n-hexane (1:2), and dried in vacuo to obtain Δ 4,6 -Androsdiene-3β, 17β-diol, white crystal, 630mg (63%, 2.2mmol); mp: 155~158℃; IR(cm-1): 3400, 2960, 1670, 1460; 1 H-NMRδ: 3.62~3.66 (1H, m, H-3), 5.60 (1H, d, J=9.8Hz, H-6), 5.96 (1H, dd, J=2.4Hz, 10.0Hz, H-7 ).
Embodiment 2
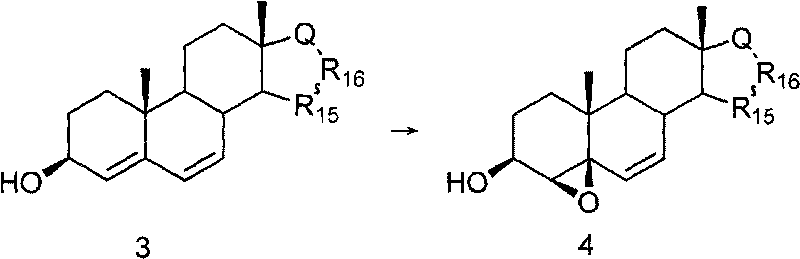
[0030] At room temperature, m-chloroperoxybenzoic acid (m-CPBA, 600 mg, 3.5 mmol) was added to Δ 4,6 - Androsdiene-3β, 17β-diol (500mg, 1.7mmol) in dichloromethane (50mL) solution, kept stirring overnight. After the reaction was completed, water (50 mL) was added and stirred for 30 min. The aqueous layer was separated, and the aqueous layer was extracted (3 x 50 mL) with dichloromethane. The organic layers were combined, washed successively with aqueous sodium sulfite (10%), aqueous sodium bicarbonate, water and brine, dried over anhydrous magnesium sulfate, filtered, concentrated, column separated or recrystallized with ethyl acetate / n-hexane (1:2) , 4β,5β-epoxy-Δ was obtained after vacuum drying 6 -Androstene-3β, 17β-diol, white crystals, 332mg (65%, 1.1mmol); mp: 146~148°C; IR (cm -1 ): 3405, 2933, 1455; 1 H-NMR δ: 3.57~3.80 (2H, m, H-3, H-17), 4.17 (1H, d, J=3.2Hz, H-4), 5.32 (1H, dd, J=2.2Hz, 6.4 Hz, H-7), 5.64 (1H, d, J = 2.2 Hz, H-6).
Embodiment 3
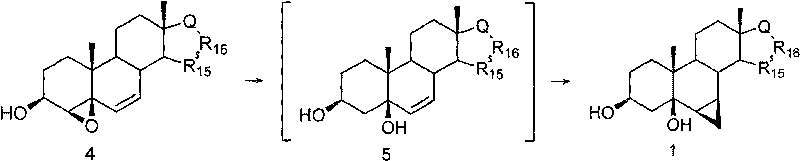
[0032] 4β,5β-epoxy-Δ 6 -Androstene-3β, 17β-diol (10.5g, 34.5mmol) was added to anhydrous THF (300mL) / LiAlH 4 (5.7g, 150mmol) in suspension. The reaction was stirred at room temperature until the reaction was completed (TLC followed the reaction, about 40 min). The reaction feed solution was placed on an ice-water bath, continued to stir, and methanol was added dropwise until no gas was released, and then 10% aqueous hydrochloric acid (1000 mL) was added with stirring. Extract with isopropyl ether (3×100mL), wash the ether layer with water successively, dry over anhydrous magnesium sulfate, remove the solvent by vacuum extraction, recrystallize with ether, filter, and dry in vacuum to obtain Δ 6 -Androstene-3β, 5β, 17β-triol, white crystalline powder, 9.8g (93%, 32.1mmol); mp: 110-113°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com