Sustained-release microsphere containing risperidone and preparation method thereof
A slow-release microsphere preparation, a technology of risperidone, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, microcapsules, etc. It is relatively expensive and other problems, and the preparation method is simple and easy to implement, the encapsulation rate is improved, and the irritation is reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of the risperidone microspheres of embodiment 1 slow-release 30 days
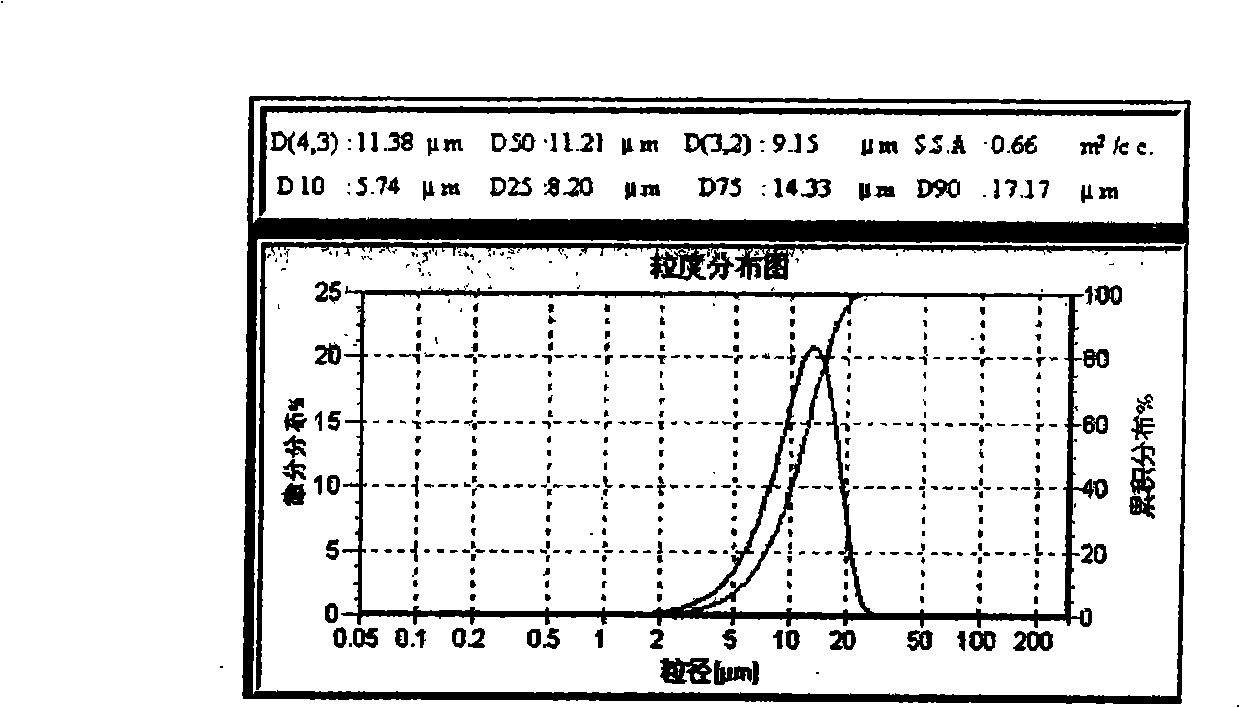
[0023] Take 1.8 g of PLGA (molecular weight 20000, LA / GA 60:40) and tert-butyl p-hydroxyanisole (BHA) dissolved in an organic solvent to form an oil phase. The organic solvent is dichloromethane (DCM) or a mixture of dichloromethane and methanol. Dissolve 500mg of risperidone sample in the above oil phase, and mix evenly by ultrasonic. Weigh an appropriate amount of PVA in double-distilled water, dissolve it fully in a water bath at 50°C, and adjust its concentration to 5% as the water phase. Inorganic salts are added to the aqueous dispersion medium solution. Inject the above oil phase into 150ml of PVA solution, stir at 1500rpm, dilute the concentration of PVA in the water phase to 1.5% after 5 minutes, and stir magnetically at room temperature for 3-5 hours to obtain the risperidone microsphere suspension. Then washed, centrifuged and dried. The average particle size is about 1...
Embodiment 2
[0024] Preparation of risperidone microspheres of embodiment 2 sustained release for 45 days
[0025] Take 1.8g of PLGA (molecular weight is 25000, LA / GA 90:10) and BHA are dissolved in organic solvent to make oily phase. The organic solvent is dichloromethane (DCM) or a mixture of dichloromethane and methanol. Dissolve 500mg of risperidone sample in the above oil phase, and mix evenly by ultrasonic. Weigh an appropriate amount of PVA in double-distilled water, dissolve it fully in a water bath at 50°C, adjust the concentration to 5% as the water phase, and add inorganic salts to the water phase dispersion medium solution. Inject the above oil phase into 150ml of PVA solution, stir at 1500rpm, dilute the concentration of PVA in the water phase to 1.5% after 5 minutes, and stir magnetically at room temperature for 3-5 hours to obtain the risperidone microsphere suspension. Then washed, centrifuged and dried. The average particle size is about 15 μm.
Embodiment 3
[0026] Example 3 Preparation of Risperidone Microspheres by Single Emulsion Method
[0027] 1.8 g of PLGA (molecular weight 20000, LA / GA 60:40) was dissolved in an organic solvent to form an oil phase. The organic solvent is ethyl acetate (DCM) or a mixture of ethyl acetate and methanol. Dissolve 500mg of risperidone sample in the above oil phase, and mix evenly by ultrasonic. Weigh an appropriate amount of PVA in double-distilled water, dissolve it fully in a water bath at 50°C, adjust the concentration to 5% as the water phase, and add inorganic salts to the dispersion medium solution of the water phase. Inject the above oil phase into 150ml of PVA solution, stir at 1500rpm, dilute the concentration of PVA in the water phase to 1.5% after 5 minutes, and stir magnetically at room temperature for 3-5 hours to obtain the risperidone microsphere suspension. After washing, centrifuging and drying, add a small amount of methionine solution and freeze-dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com