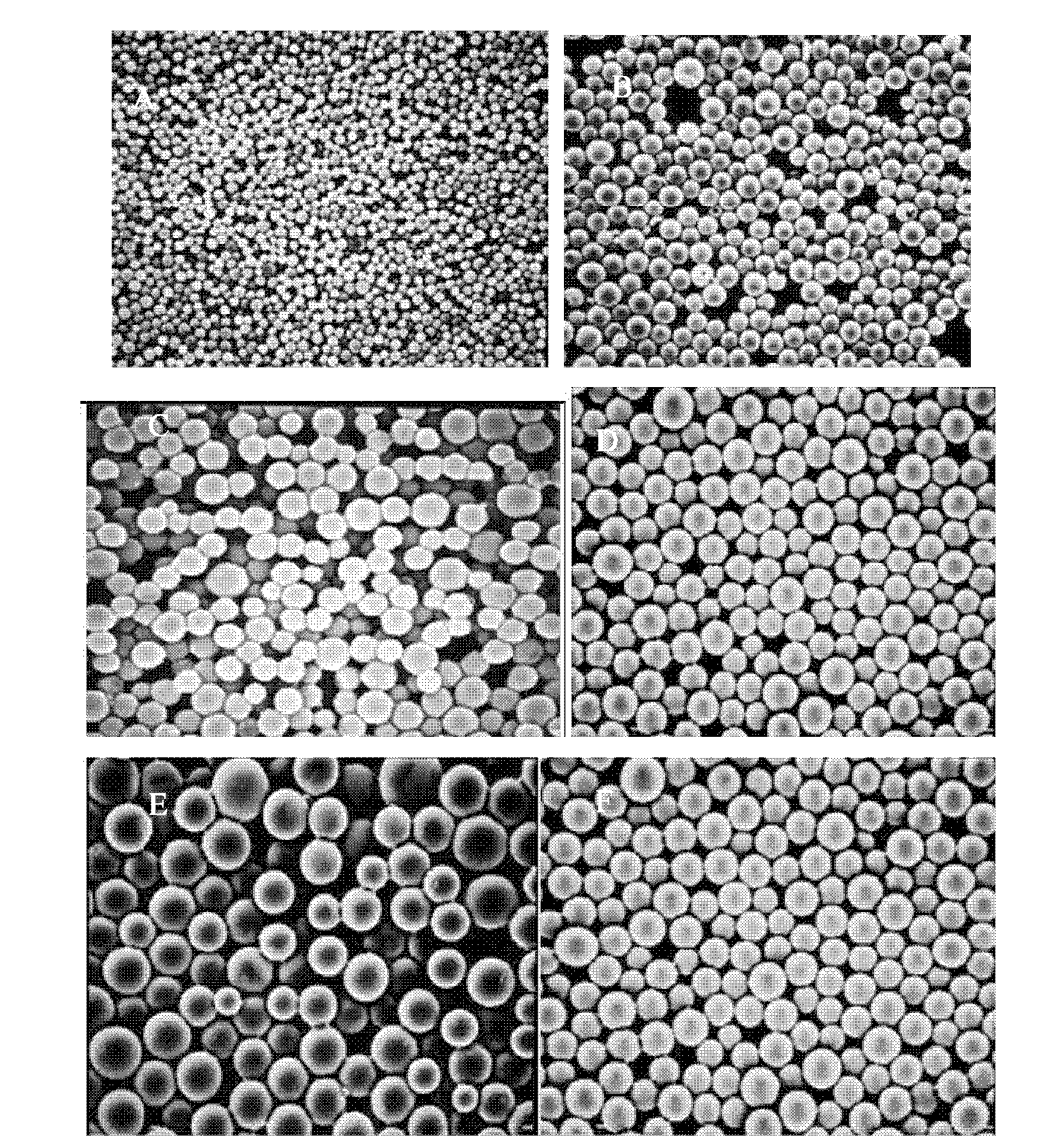
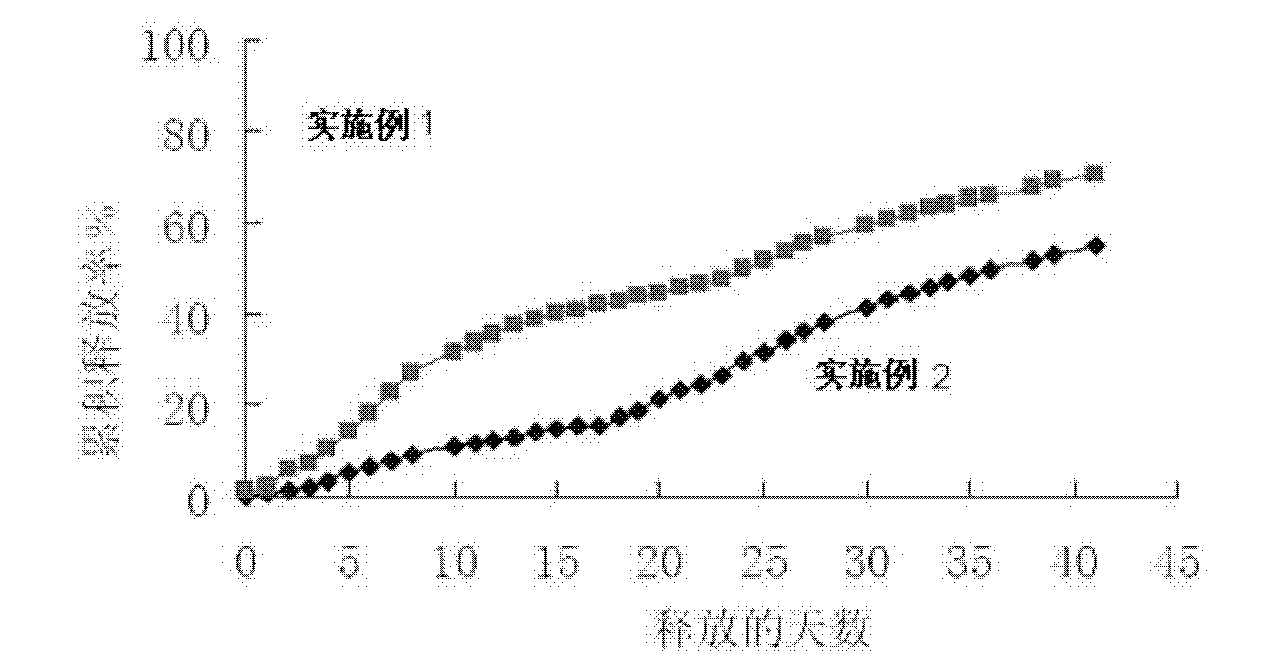
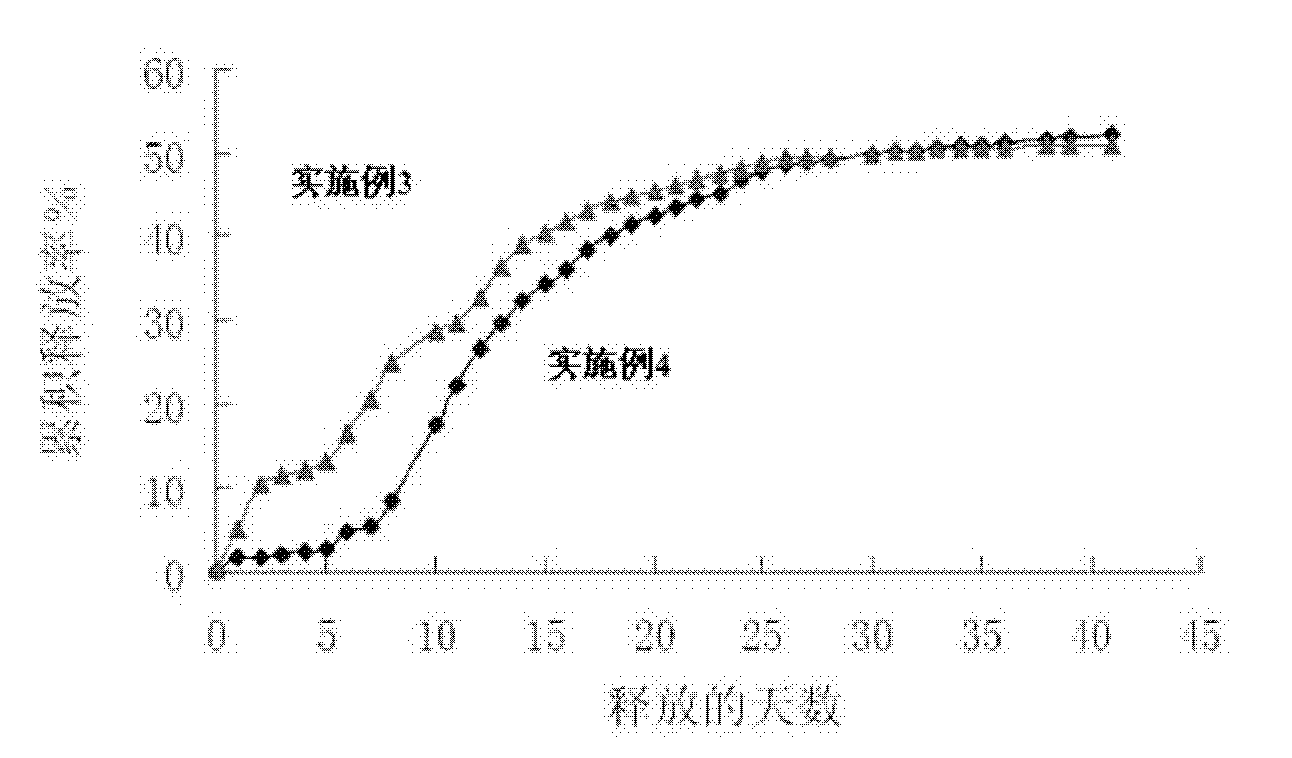
Microsphere preparation method for adjusting and controlling release behavior of risperidone microspheres and for controlling size thereof
A technology of risperidone and microspheres, which is applied in the fields of nervous system diseases, powder delivery, drug combination, etc., can solve the problems that the encapsulation rate is difficult to control, the problem of particle size cannot be overcome, and there is no innovation, so as to achieve good redispersibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] ① Weigh a certain amount of PLGA or PLA and add it to 1g of dichloromethane, vortex to dissolve, and set aside;
[0036] ② Weigh 50 mg of risperidone and add it to shake to dissolve, then suspend an appropriate amount of alkali in this solution to obtain a suspension;
[0037] ③Then transfer the suspension to the SPG film emulsion instrument, select a film emulsion with a pore size of 19.96 μm, adjust the pressure, and transfer the prepared microspheres into 1L of 4%PVA+5%NaCl solution by weight, to Stir and solidify at a speed of 150 rpm for 3 hours.
[0038] ④Use a clean vial to collect the microspheres that have completed step ③, wash them 5 times with distilled water, put them in a -2°C refrigerator overnight, and then take them out and vacuum freeze-dry them for 24 hours at a pressure lower than 0.05mbar.
[0039] According to the degradation cycles of PLGA and PLA with different molecular weights provided by Lakershore Company, high-viscosity PLA (IV=0.71, MW=109...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com