Kangfuxin solution preparation fingerprint quality determination method and standard fingerprint
A technology of Kangfuxin liquid and fingerprints, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficult to effectively control the quality of Kangfuxin liquid preparations, chromatographic peak shapes and poor resolution, and achieve Kangfuxin liquid Accurate and objective quality control of preparations, rapid identification, and good separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0020] (1) Instruments and reagents
[0021] TSQ Quantum Ultra liquid chromatography-tandem mass spectrometer; Hypersil Gold C18 column (4.6×150mm, 5μm).
[0022] Methanol is chromatographically pure, water is ultrapure water, and acetic acid is analytically pure.
[0023] 10 different batches of rehabilitation new liquid: batch number 140308, batch number 140309, batch number 140310, batch number 140311, batch number 140312, batch number 140313, batch number 140314, batch number 140315, batch number 140316, batch number 140317.
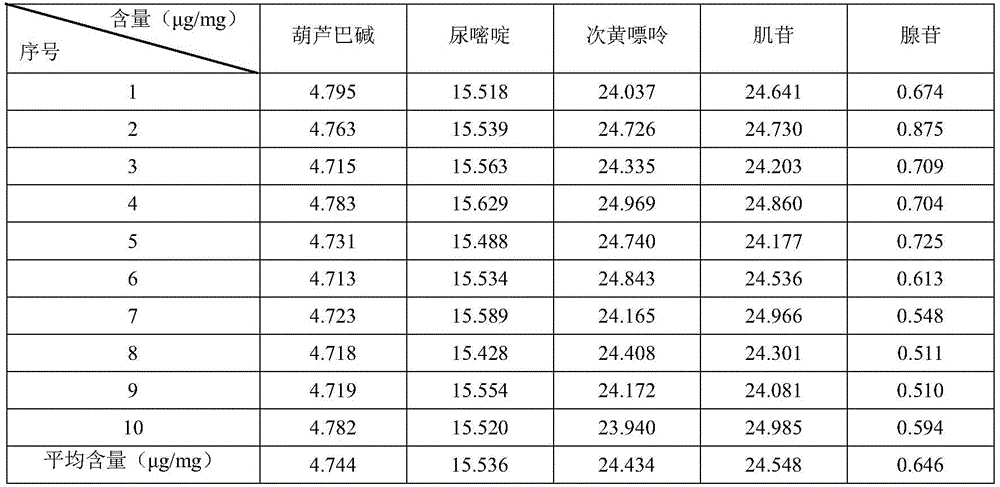
[0024] Trigonelline, uracil, hypoxanthine, adenosine, and inosine (all purchased from China Pharmaceutical and Biological Products Laboratory, batch numbers are A0654, PCS3048, PCS3055, PCS0217, PCS3058).
[0025] (2) Chromatographic conditions
[0026] a. Selection of detection wavelength
[0027] Since Kangfuxin Liquid is an alcohol extract of the medicinal insect Periplaneta americana, it contains a large amount of nucleoside substances, and existing studies ...
Embodiment 2
[0087] The quality measurement method of the fingerprint of Kangfuxinye preparation includes the following steps:
[0088] (A) Preparation of the test solution: take the Kangfuxin liquid preparation, pass through a 0.22μm microporous membrane, take the additional filtrate, and set aside;
[0089] (B) Preparation of reference solution: accurately weigh trigonelline, uracil, hypoxanthine, inosine, adenosine, and add water to make each 1mL containing trigonelline, uracil, hypoxanthine, inosine and Adenosine is a mixed control solution of 20μg, 30μg, 20μg, 40μg and 40μg, for use;
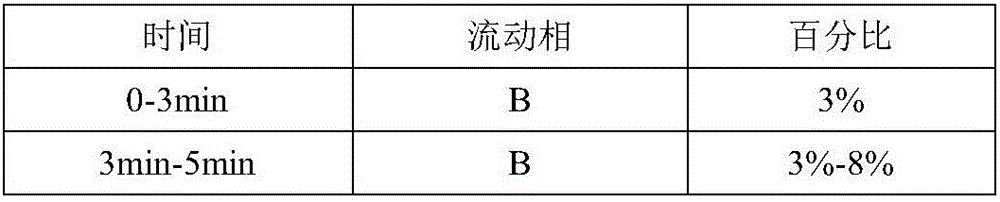
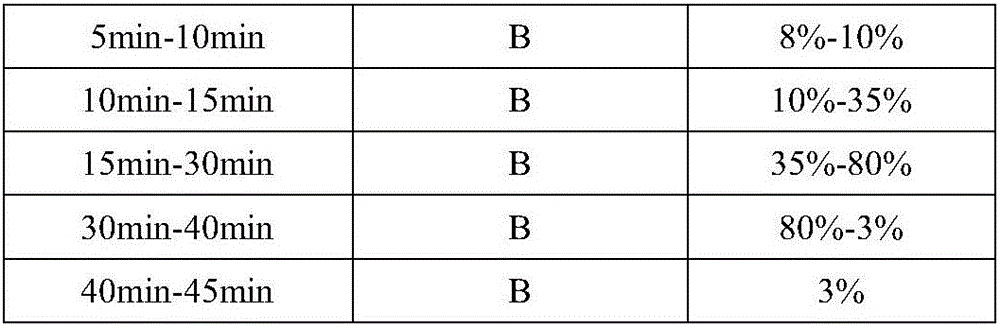
[0090] (C) Determination: Inject 20μL each of the test product and the reference solution into the high performance liquid chromatograph, use 0.08% acetic acid aqueous solution (A)-methanol (B) as the mobile phase, and use gradient elution to obtain the Kangfu Xinye preparation Fingerprint.
[0091] The measurement conditions of high performance liquid chromatograph are: filler is octadecylsilane bonded silica...
Embodiment 3
[0094] The quality measurement method of the fingerprint of Kangfuxinye preparation includes the following steps:
[0095] (A) Preparation of the test solution: take the Kangfuxin liquid preparation, pass through a 0.22μm microporous membrane, take the additional filtrate, and set aside;
[0096] (B) Preparation of reference solution: accurately weigh trigonelline, uracil, hypoxanthine, inosine, adenosine, and add water to make each 1mL containing trigonelline, uracil, hypoxanthine, inosine and Adenosine is a mixed control solution of 20μg, 30μg, 20μg, 40μg and 40μg, for use;
[0097] (C) Determination: Inject 20μL each of the test product and the reference solution into the high performance liquid chromatograph, use 1.0% acetic acid aqueous solution (A)-methanol (B) as the mobile phase, and use gradient elution to obtain the Kangfu Xinye preparation Fingerprint.
[0098] The measurement conditions of high performance liquid chromatograph are: filler is octadecylsilane bonded silica ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com