Preparation method of liposome of medicine composition of ceftriaxone sodium and sulbactam sodium
A technology of ceftriaxone sodium and sulbactam sodium, which can be used in liposome delivery, pharmaceutical formulations, active ingredients of heterocyclic compounds, etc., and can solve problems such as poor absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
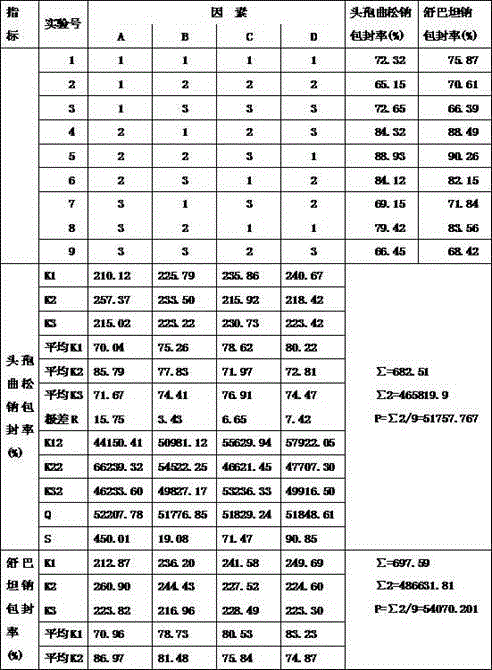
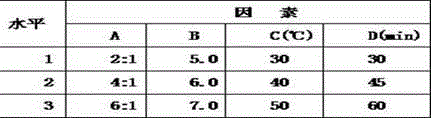
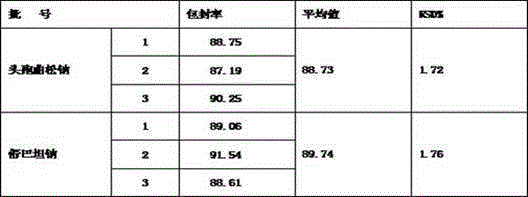
[0020] Set forth the present invention with example below:
[0021] Case number one:
[0022] Ceftriaxone Sodium 100g Sulbactam Sodium 50g Soy Lecithin 100 cholesterol 33g Sodium dihydrogen phosphate 1.6g Disodium phosphate 0.3g production 100 bottles
[0023] Preparation process: Take the prescribed amount of soybean lecithin and cholesterol and dissolve them completely in 230ml of absolute ethanol. or mixed.) Remove absolute ethanol on a rotary evaporator to obtain a phospholipid film.
[0024] Weigh 0.3 g of disodium hydrogen phosphate and 1.6 g of sodium dihydrogen phosphate and add it to 800 ml of water to dissolve completely, prepare a phosphate buffer solution (PBS) with a pH of about 5.8, add it to the phospholipid membrane for hydration, and use a high-pressure homogenizer to prepare a blank lipid body.
[0025] Weigh ceftriaxone sodium and dissolve it in 800ml of water, filter it at 0.45um after dissolving complet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com