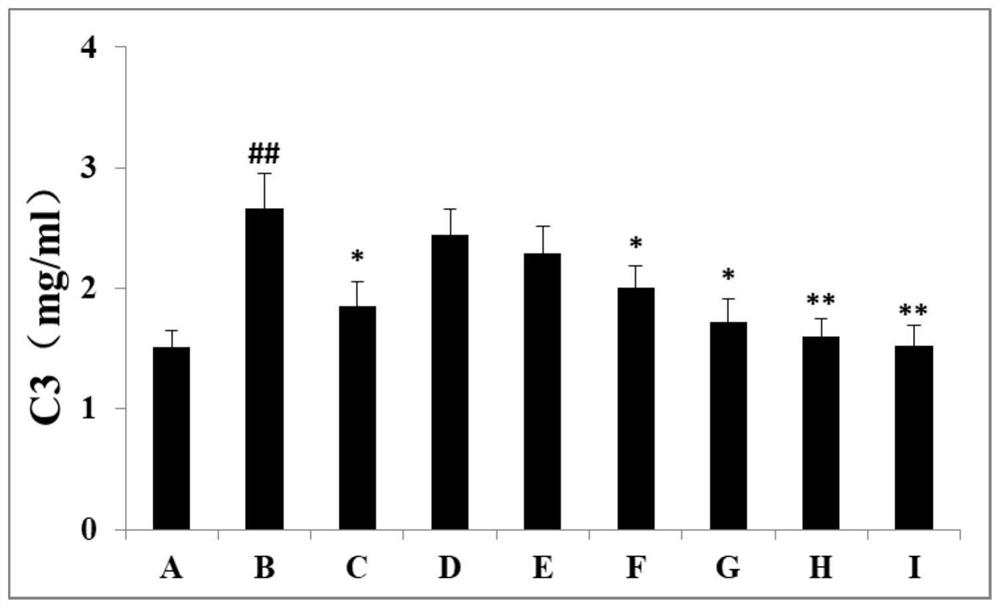
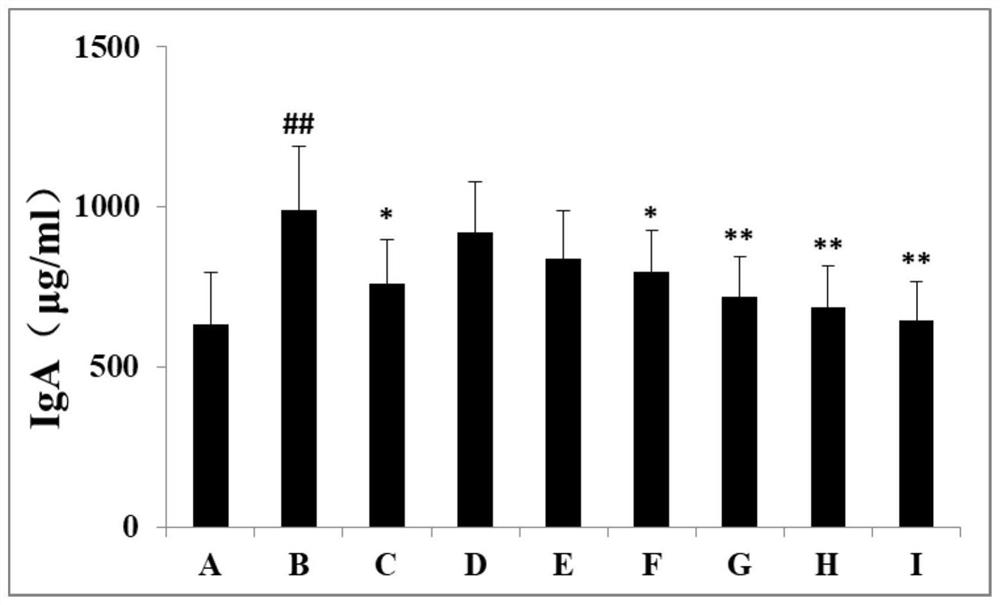
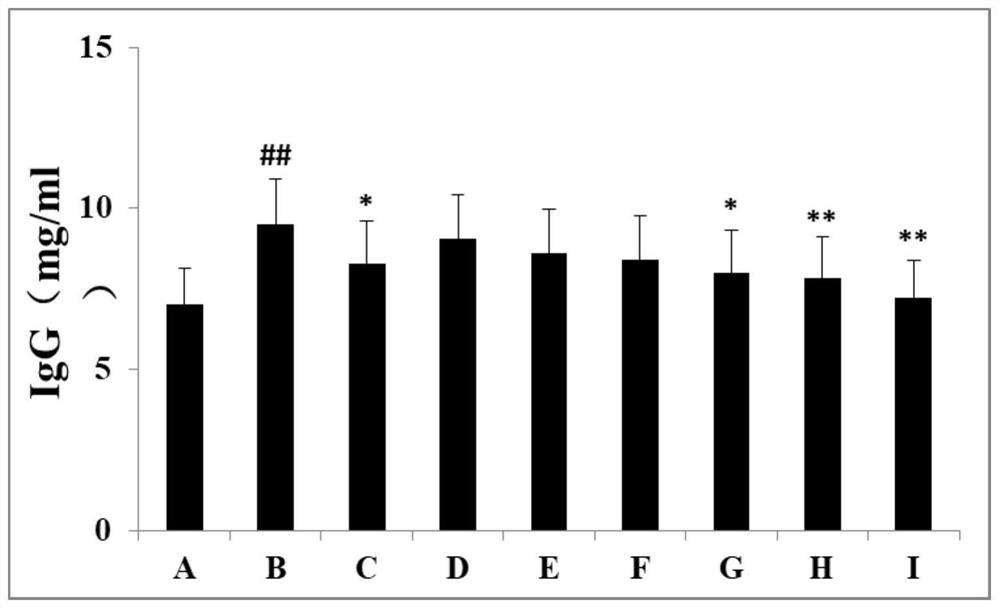
Patents
Literature
47 results about "Sodium ceftriaxone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
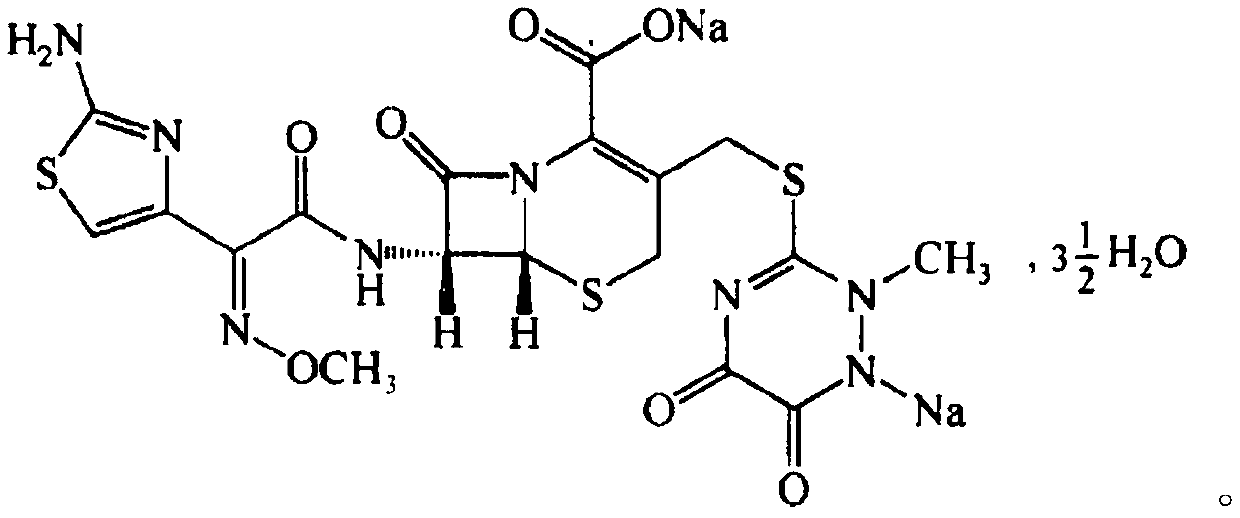
Ceftriaxone (ceftriaxone sodium and dextrose) Injection is an antibacterial drug used to treat conditions such as lower respiratory tract infections, skin and skin structure infections, urinary tract infections, pelvic inflammatory disease, bacterial septicemia, bone and joint infections, and meningitis.
Crystal form of ceftriaxone sodium and preparation method for crystal form
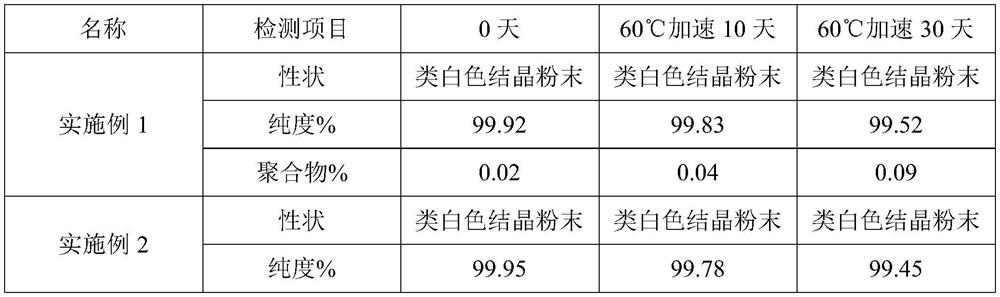
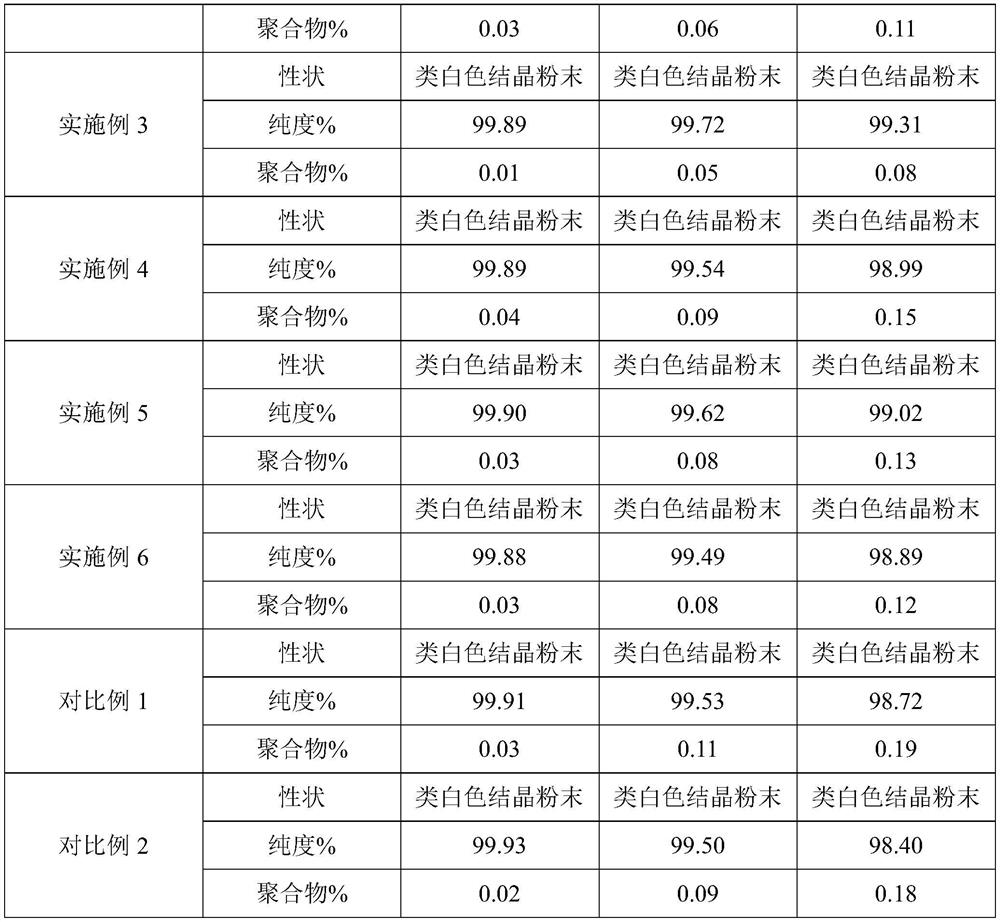
InactiveCN102875574AMeet the requirementsImprove stabilityOrganic chemistryCeftriaxonumSodium ceftriaxone
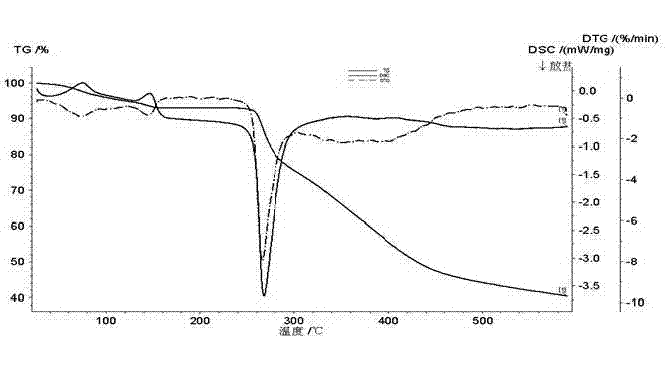
The invention discloses a novel crystal form of ceftriaxone sodium. The content of the novel crystal form is over 94 percent, and the novel crystal form is high in stability and accords with the medicinal standard. The invention also discloses a preparation method for the novel crystal form. By the method, a seed crystal is not required to be added, the preparation method is simple in operation, the yield is over 84 percent, and the novel crystal form is suitable for industrial production.
Owner:石药集团中诺药业(石家庄)有限公司
Ceftriaxone sodium powder-injection for injection
ActiveCN104873466ALess impuritiesImprove stabilityAntibacterial agentsPowder deliveryNorth chinaSolvent
The invention discloses a ceftriaxone sodium powder-injection for injection. the powder-injection is prepared by the following steps: (1) weighing a ceftriaxone sodium crude raw material at 20 DEG C, adding water, stirring and dissolving, adding active carbon, decolouring, filtering, and washing with mixed solvents; (2) by a particle process crystal product molecular assembly and shape-state optimization technology of North China Pharmaceutical Hebei Huamin Pharmaceutical Co., Ltd., adding a solventing-out agent acetone according to a stream acceleration table at 15 DEG C at the stirring speed of 300 r / min; (3) carrying out suction filtration, washing a filter cake with acetone, putting the filter cake into a vacuum drying oven and carrying out vacuum drying at 30-40 DEG C; and (4) sub-packaging preparations of different specifications, and controlling environmental temperature and humidity until temperature is 20-24 DEG C and humidity is less than 40% so as to obtain the ceftriaxone sodium for injection. In comparison with a traditional technology, ceftriaxone sodium prepared by the above preparation method has advantages of less impurity, high stability and the like.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA +1
Original-quality ceftriaxone sodium and pharmaceutical preparation thereof
ActiveCN105418641AHarm reductionToxicAntibacterial agentsOrganic active ingredientsChloroformateAntibiotic Y
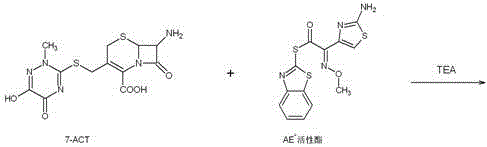
The invention discloses original-quality ceftriaxone sodium and a pharmaceutical preparation thereof. The key technology and industrialization of the third generation of cephalosporin antibiotics active ester intermediate wins the second prize of National Scientific and Technological Progress Award, and the third generation of cephalosporin antibiotics intermediate AE active ester is a key factor for affecting the internal quality of the ceftriaxone sodium. A preparation method comprises the steps that 1, boron trifluoride-acetonitrile serves as a catalyst, and on the condition that acetonitrile serves as solvent, a triazine ring is reacted with 7-ACA to generate 7-ACT; 2, triethylamine and aminothiazoly loximate are added into the solvent, a chloroformate activator is dropwise added slowly during cooling mixing, the 7-ACT is added for a one-pot reaction after stirring is conducted, and ceftriaxone is obtained; 3, a salt-forming agent is added, and the ceftriaxone sodium is obtained. According to the preparation method, use of a condensing agent with higher price is avoided, the process route is shortened, operation is easy, the reaction condition is mild, the product yield is high, the purity is good, and industrial production is easy.
Owner:广东金城金素制药有限公司 +1
Ceftriaxone sodium compound entity for children and preparation for ceftriaxone sodium compound entity for children
InactiveCN104926834AMaintain stabilityIncrease internal pressureAntibacterial agentsPowder deliveryActivated carbonTemperature control
The invention provides a ceftriaxone sodium compound entity for children. A structural formula of the ceftriaxone sodium compound entity is as follows: formula as shown in the specification. The ceftriaxone sodium compound entity is prepared by the following steps: (1) dissolving a ceftriaxone crude product into water, adding activated carbon, stirring and discoloring, and filtering; (2) adding an extraction agent into filtrate to obtain a mixture, transferring and filling the mixture into a pressure-resistant container, and carrying out temperature-controlled freezing on the mixture and taking out the mixture after removing air bubbles; and (3) removing an organic phase of the mixture, dropwise adding acetone at 10-15 DEG C after solids are molten, slowly stirring, growing crystals, filtering, washing, carrying out vacuum drying and packaging preparations of different specifications. Compared with ceftriaxone sodium prepared by a conventional process, the ceftriaxone sodium prepared by the preparation method has the advantages of few impurities, high stability and the like.
Owner:ZHEJIANG CHANGDIAN PHARMA
Preparation method and product of ceftriaxone sodium sterile powder
InactiveCN106008554AAvoid open loopAvoid degradationAntibacterial agentsPowder deliveryCeftriaxonumSodium ceftriaxone
The invention discloses a preparation method of ceftriaxone sodium sterile powder, and belongs to the technical field of medicine. The method comprises the following steps of mixing methylene dichloride and alcohol; lowering the temperature; sequentially adding antioxidizers, 7-ACT and AE-active esters; dissolving the materials to obtain a mixed solution; dripping triethylamine into the mixed solution within 1h for reaction; performing extraction after the reaction is completed; after the pressure reduction suction filtration on a water phase, adding a salt forming agent for reaction and salt forming; after a decoloring agent is added into a salt forming reaction solution, performing sterile filtration; adding a solvating agent into filter liquid for crystallization; performing post-treatment to obtain the ceftriaxone sodium sterile powder. The preparation method has the advantages that the operation is simple; the reaction conditions are mild; the control is easy; the ceftriaxone sodium sterile powder is prepared in one step; the refining process is omitted; the production period is shortened; the cost is greatly reduced; the quality yield reaches 165 percent or higher; the product purity can reach 99.5 percent or higher.
Owner:HENAN KANGDA PHARMA
Preparation method of liposome of medicine composition of ceftriaxone sodium and sulbactam sodium
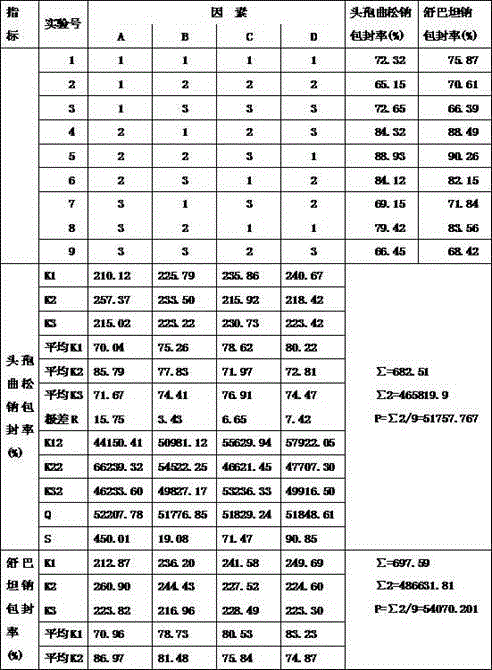
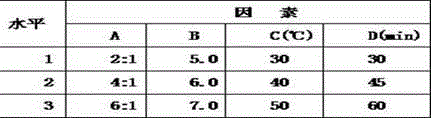
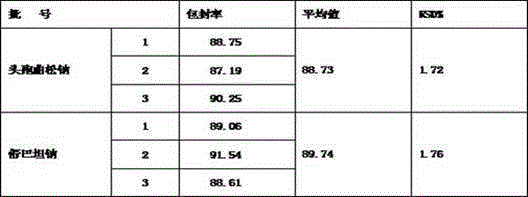
ActiveCN102973568APromote crystallizationHigh dosageAntibacterial agentsLiposomal deliveryDrugSulbactam Sodium
The invention relates to a preparation method of liposome of a medicine composition of ceftriaxone sodium and sulbactam sodium. According to the method, the liposome is prepared in a combining form of medicines, and the specification proportion of the ceftriaxone sodium to the sulbactam sodium is equal to (1-4):1. The preparation method comprises the following main steps of: preparing blank liposome from phospholipid and cholesterol, preparing the liposome from the ceftriaxone sodium and the sulbactam sodium which are respectively subjected to passive loading, and performing freeze drying on the prepared liposome to obtain a finished product; or dissolving the ceftriaxone sodium and the sulbactam sodium in phosphate buffer with the pH ranging from 6.0 to 6.5, and adding phospholipid cholesterol and a freeze-dried support agent to the solution in an active loading manner to prepare a freeze-drying preparation of the liposome.
Owner:JIANGSU SKYRUN PHARMA CO LTD
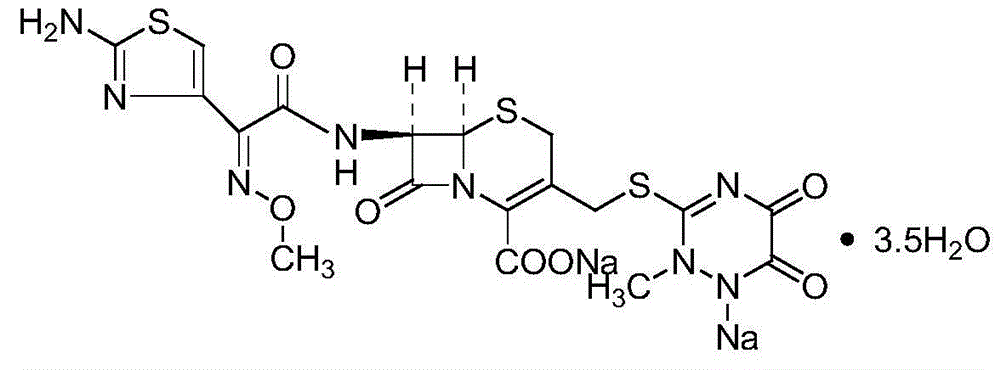
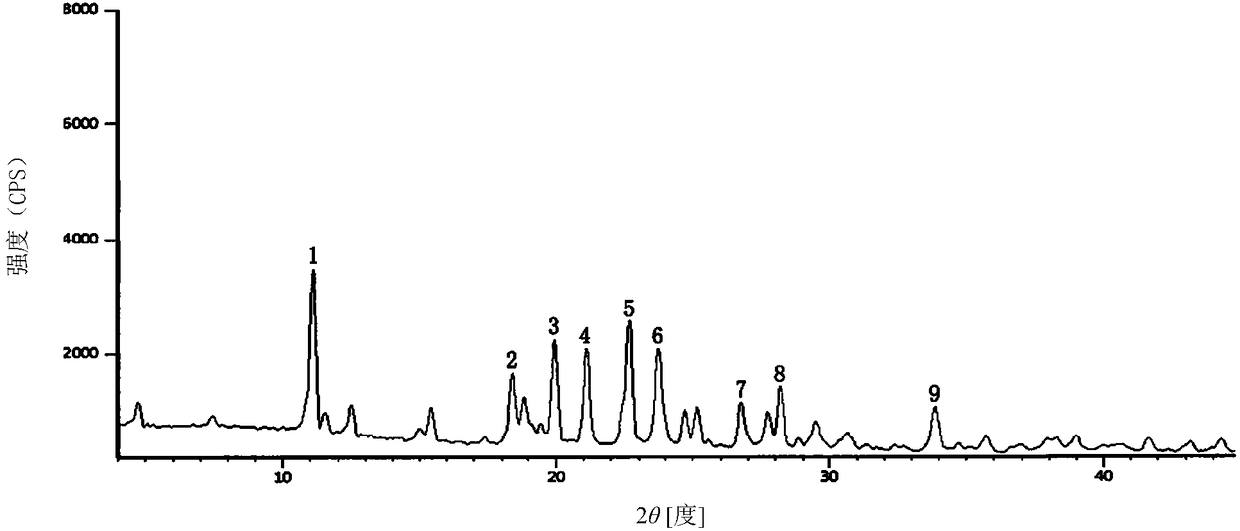
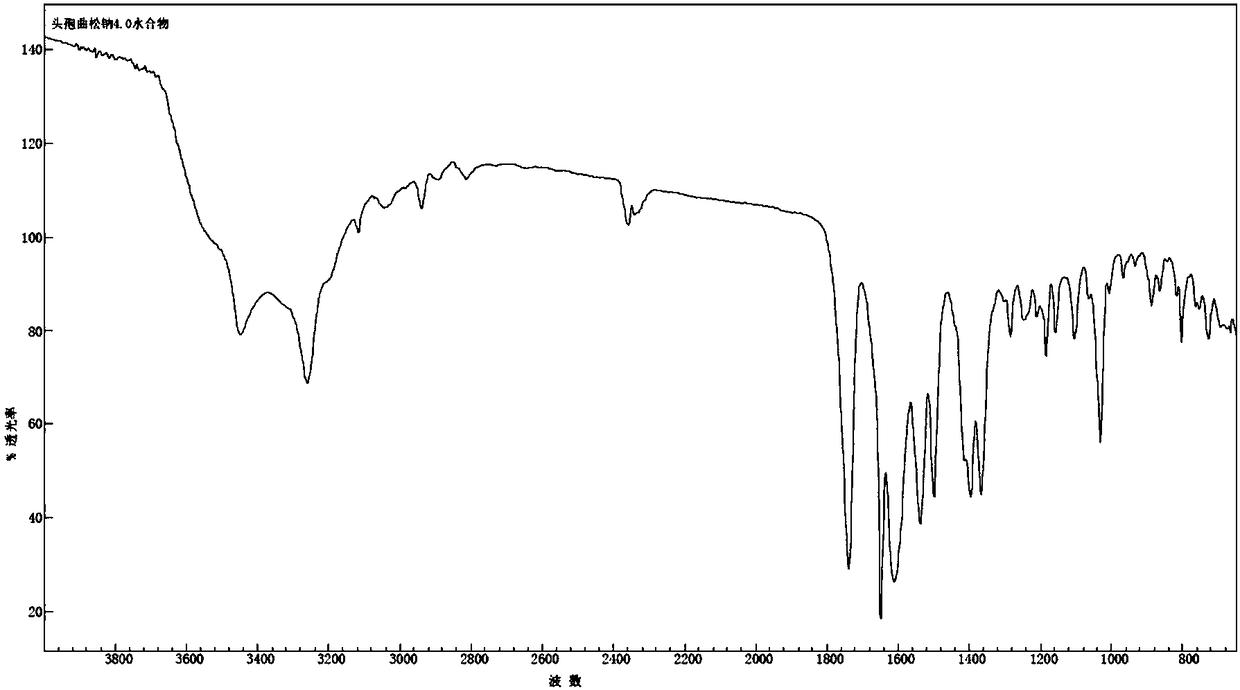
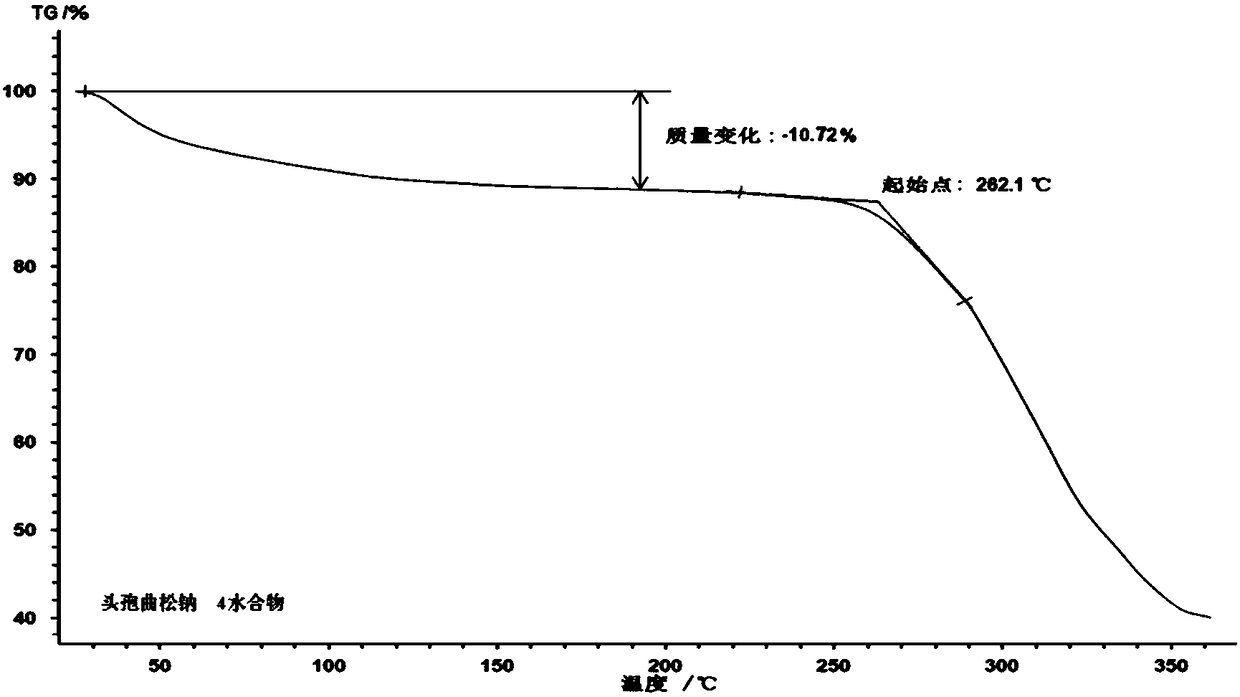
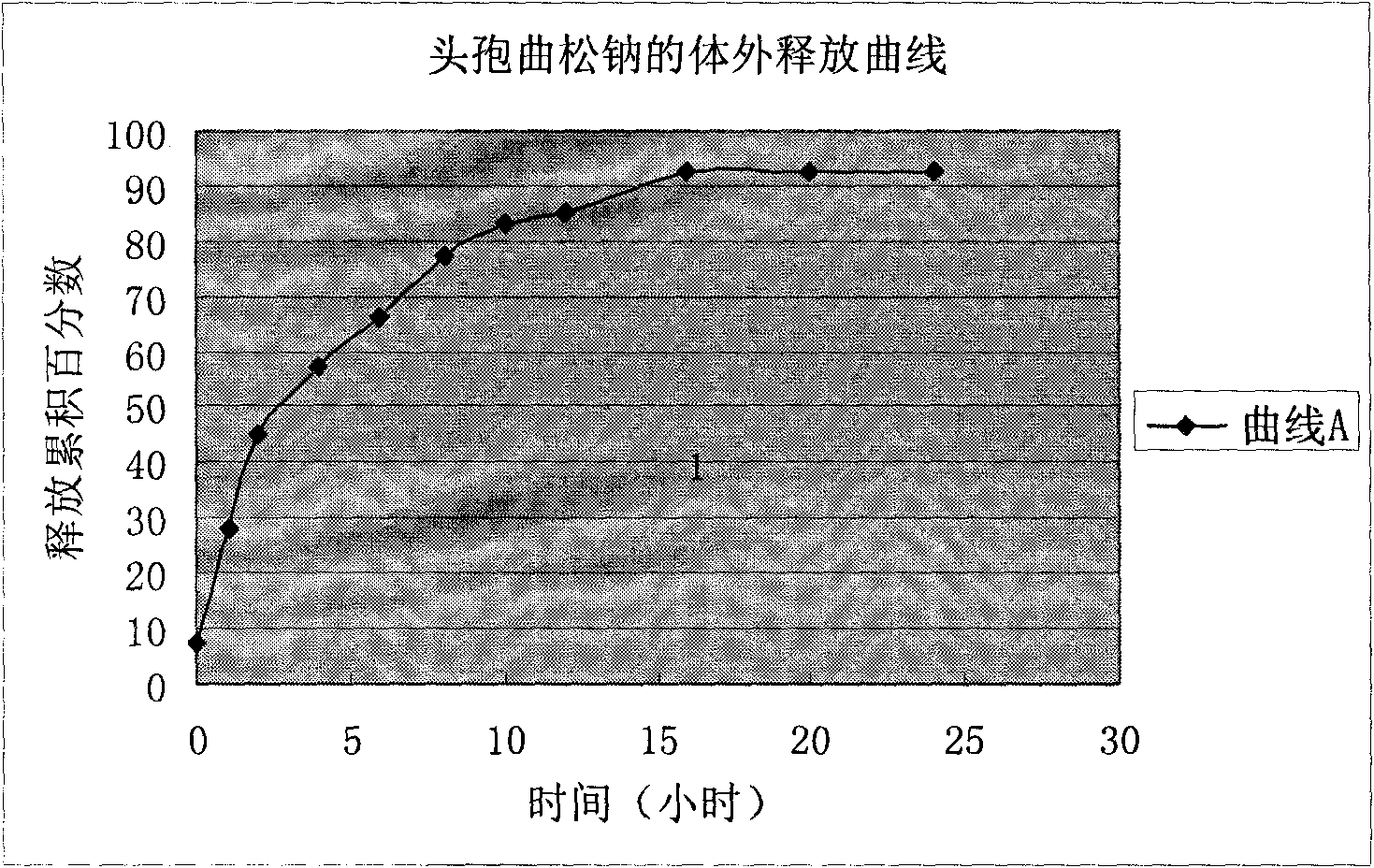
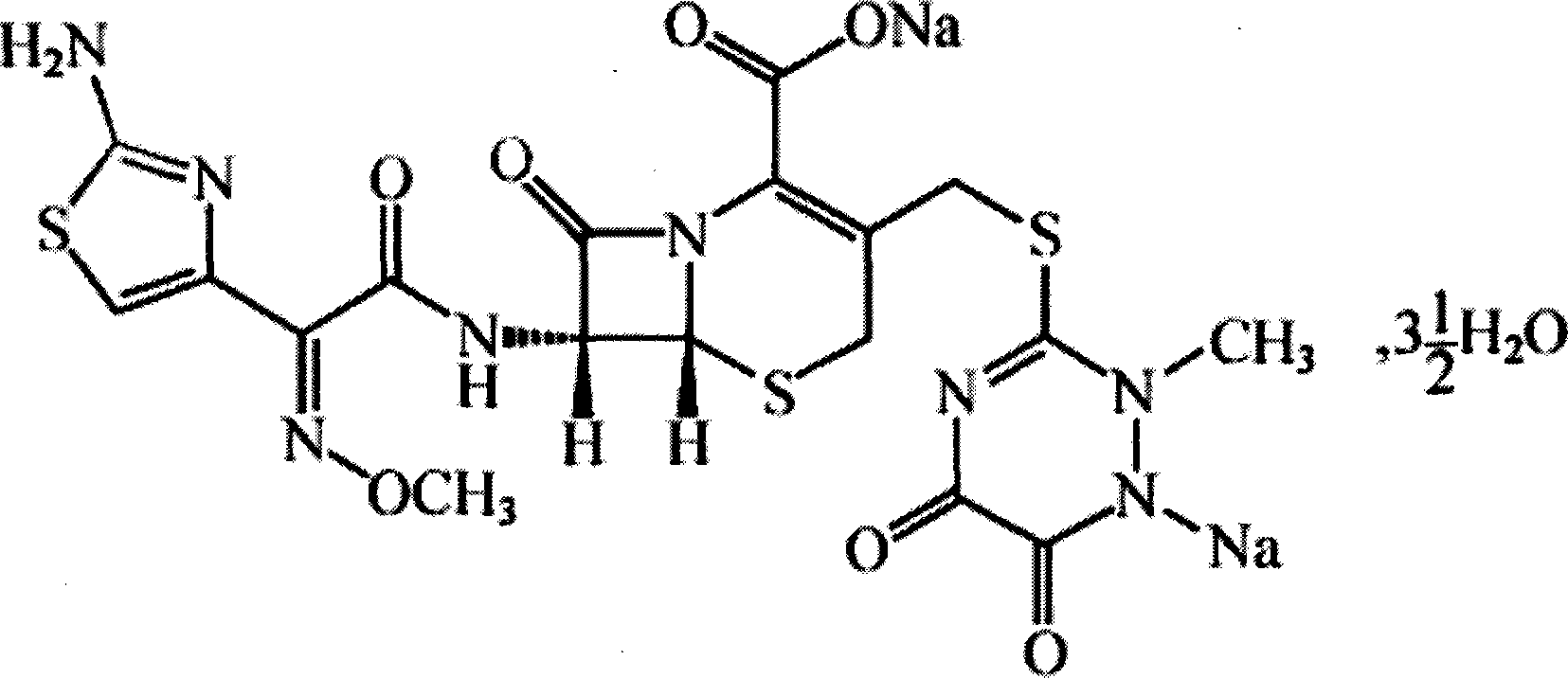
Ceftriaxone sodium tetrahydrate compound
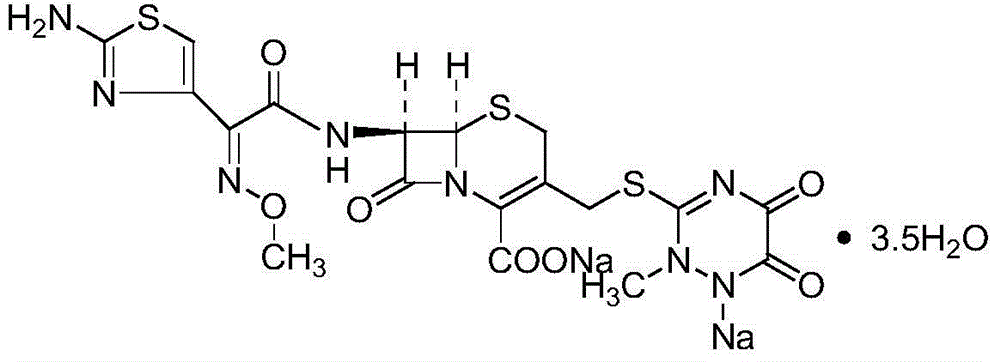
The invention discloses a ceftriaxone sodium tetrahydrate compound and a preparation method thereof. Each mole of ceftriaxone sodium contains four mole of water. According to the compound, sulfochlorides and dimethylformamide are adopted to be reacted to generate an activator, the activator is directly reacted with 7-aminoceftriazine tetramethylguanidine salt to obtain the ceftriaxone sodium tetrahydrate compound. The operation is simple, the obtaining of a reactant is easy, the reaction condition is milder, and the yield is high. The ceftriaxone sodium tetrahydrate compound has the advantagesof low hygroscopicity, low impurity content, good fluidity, good thermodynamic stability and more extensive application prospects.
Owner:陕西顿斯制药有限公司 +2
Chinese and western composite medicine for treating neoplastic diseases
InactiveCN102949712AToxic reductionEasy drainage and exclusionOrganic active ingredientsPeptide/protein ingredientsSide effectAdditive ingredient
A Chinese and western composite medicine for treating neoplastic diseases is characterized by comprising Chinese herbal medicinal ingredients and a western medicinal ingredient which are mixed, the Chinese herbal medicinal ingredients include 5 polyzyme tablets and a stomach invigorating and digestion aiding tablet, each polyzyme tablet comprises pepsin, trypsin, pancreatic lipase and amylopsin, and the stomach invigorating and digestion aiding tablet comprises radix pseudostellariae, tangerine peels, Chinese yam, (roasted) malt and hawthorn; the western medicinal ingredient is ceftriaxone sodium injection solution; and the weight of each polyzyme tablet is 0.313g, the total weight of the polyzyme tablets is 1.57g, the weight of the stomach invigorating and digestion aiding tablet is 0.8g, the weight of the ceftriaxone sodium injection solution is 0.75g, and the Chinese herbal medicinal ingredients and the western medicinal ingredient are combined to form the prescription. The Chinese and western composite medicine for treating the neoplastic diseases is particularly used for suppressing and killing pathogenic bacteria capable of causing benign tumor and malignant tumor infection of tissue cells of a human body, the composite medicine is directly dripped into the oral cavity or an affected part of the human body when the human body suffers from the tumor infection, and 3-4 drops of the composite medicine are applied every time. The Chinese and western composite medicine has few side effects, becomes effective within 30 minutes and does not injure the liver, the brain and the kidneys of the human body.
Owner:李红彬
Microsphere injection of ceftriaxone sodium/tazobactam sodium drug composition
InactiveCN101804060AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsGranular deliveryDrugChemistry
The invention discloses microsphere injection of a ceftriaxone sodium / tazobactam sodium drug composition, which comprises ceftriaxone sodium, tazobactam sodium, PLA, sodium glycocholate, PEG600 and trehalose. In the preferential technical scheme, the microsphere injection comprises 3 parts of ceftriaxone sodium, 1 part of tazobactam sodium, 2.5-7 parts of PLA, 3-6 parts of sodium glycocholate, 2-5 parts of PEG600 and 8-15 parts of trehalose. Compared with the prior art, the invention has the advantages of good stability, high encapsulation efficiency, preparation process suitable for industrial production and proper slow-release effect.
Owner:HAINAN MEIDA PHARMA
Ceftriaxone sodium compound prepared by adopting supermolecular mechanism and preparation thereof
InactiveCN106317078AReduce humidityLow impurity contentAntibacterial agentsPowder deliveryCrystal systemSpace group
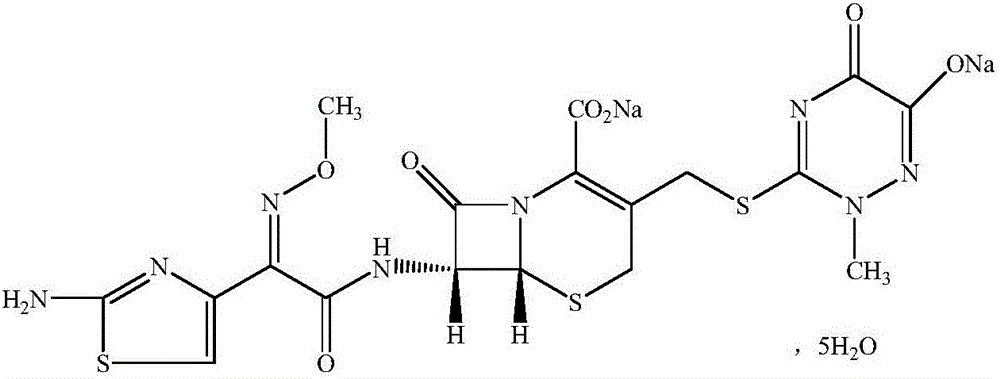
The invention discloses a ceftriaxone sodium compound prepared by adopting a supermolecular mechanism and a preparation thereof, namely ceftriaxone sodium for injection. The 'high-end medicine product refining crystallization technology development and industrialization project' won the second award for national scientific and technological progress in 2015, and the supermolecular mechanism belongs to one of the high-end medicine product refining crystallization technology. According to the structure of the ceftriaxone sodium, five water molecules are contained in the molecule, the crystal system is a monoclinic system, and the space group is P21 / c. The obtained ceftriaxone sodium is low in hygroscopicity, low in impurity content, good in product flowability and stability and high in dissolution rate.
Owner:海南顿斯医药科技有限公司 +1
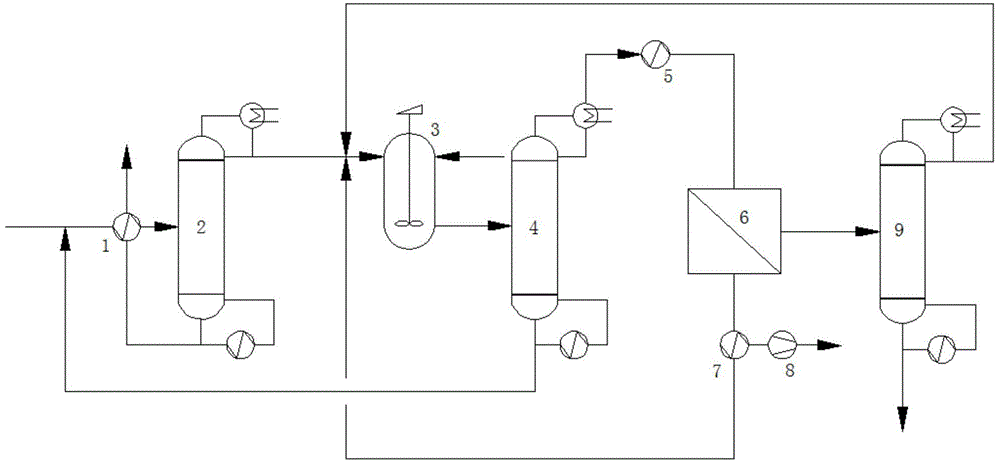
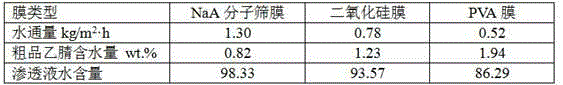
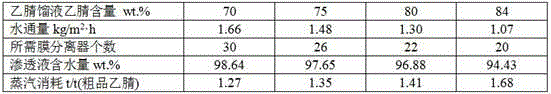
Method and device for recycling and refining acetonitrile in ceftriaxone sodium synthesis
ActiveCN104926690ASimple processImprove securityCarboxylic acid nitrile purification/separationSodium ceftriaxonePhysical chemistry
The invention relates to a method and device for recycling and refining acetonitrile in ceftriaxone sodium synthesis. The method includes the following steps: acetonitrile waste liquid in ceftriaxone sodium synthesis is fed into a first rectifying tower, and parts of water and heavy components are removed in a rectifying mode; rectified acetonitrile liquid is obtained after concentrating is carried out and fed into a neutralization tank for pH value adjustment; the neutralized rectified acetonitrile liquid is fed into a second rectifying tower for further impurity removing, the rectified acetonitrile liquid enters a pervaporation membrane separation unit after impurities are removed in the second rectifying tower, residual liquid in a kettle is returned to the first rectifying tower, and the acetonitrile in the residual liquid is recycled; the crude acetonitrile is obtained after the liquid is separated through the pervaporation membrane separation unit, water and a small amount of acetonitrile in a solution on the feed liquid side permeate a pervaporation membrane in a steam mode to obtain permeating liquid, the permeating liquid is condensed to be returned to the neutralization tank, and the acetonitrile in the permeating liquid is recycled; the crude acetonitrile obtained through the pervaporation membrane separation unit is fed into a third rectifying tower to be refined, the finished acetonitrile is obtained, acetonitrile-water azeotrope obtained after rectifying of the third rectifying tower is returned to the second rectifying tower, and the acetonitrile in the acetonitrile-water azeotrope is recycled.
Owner:JIANGSU NINE HEAVEN HIGH TECH
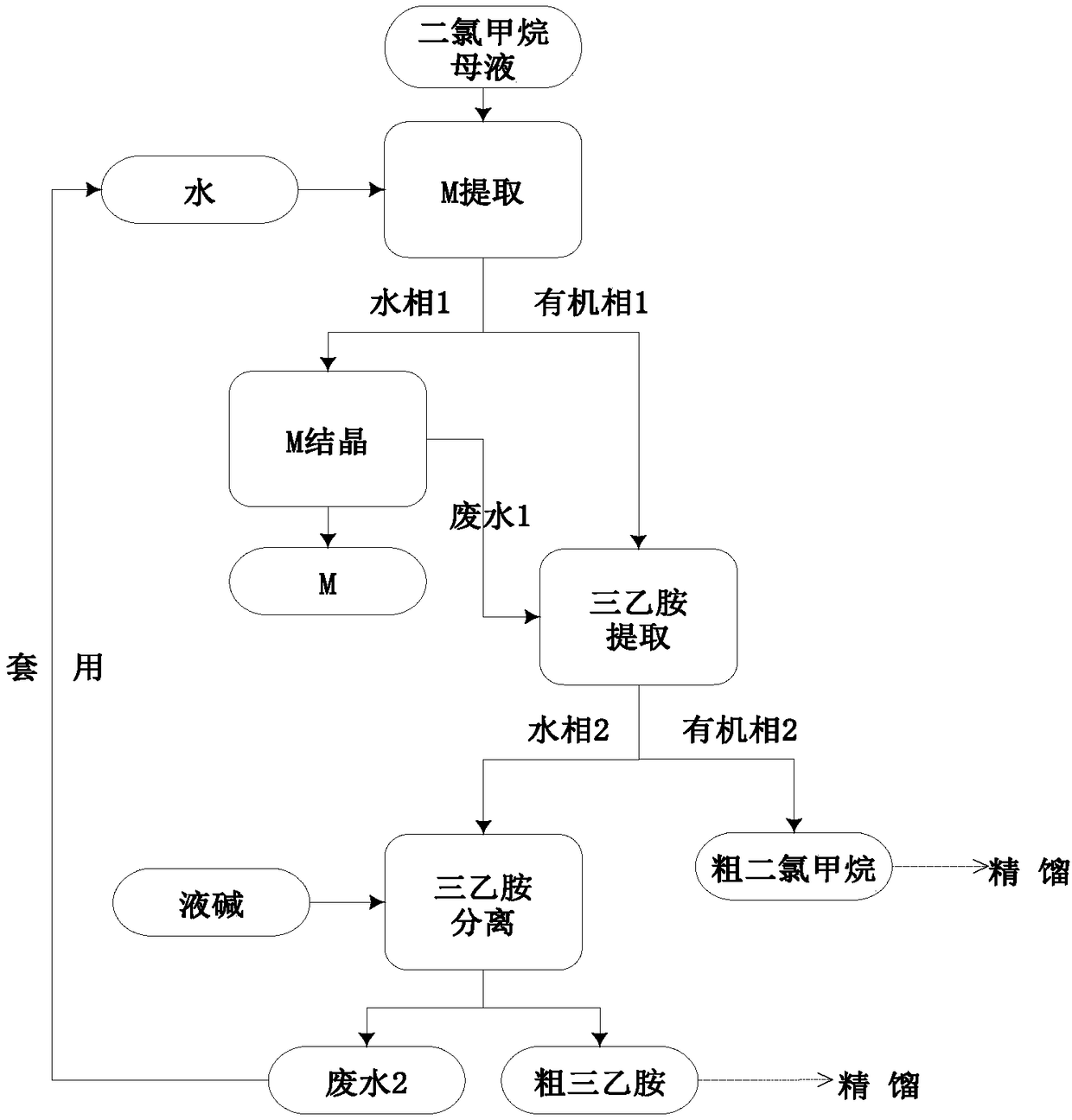
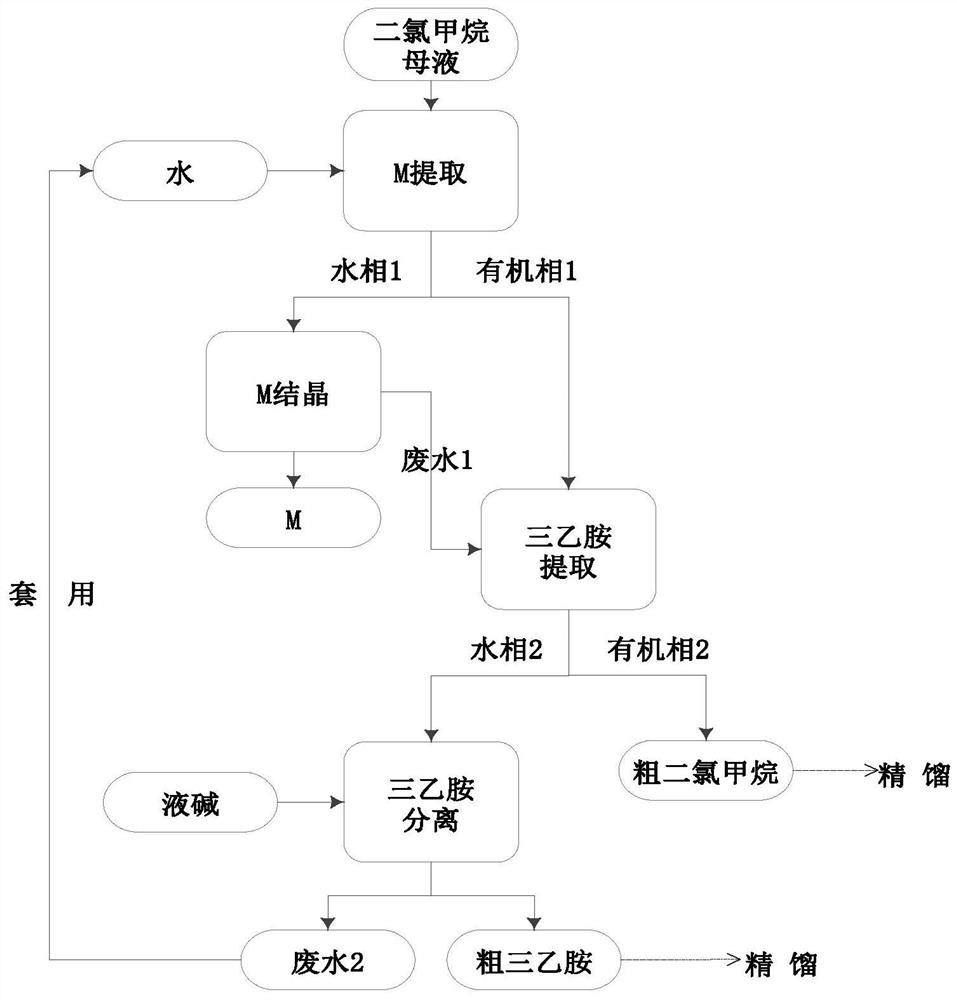
Comprehensive recycling method of 2-mercaptobenzothiazole, triethylamine, and dichloromethane in ceftriaxone sodium dichloromethane mother liquid
ActiveCN109053627AMeet the requirements of production recyclingReduce dosageAmino compound purification/separationHalogenated hydrocarbon preparationThiazoleSodium ceftriaxone
The invention discloses a comprehensive recycling method of 2-mercaptobenzothiazole, triethylamine, and dichloromethane in a ceftriaxone sodium dichloromethane mother liquid. The method provided by the invention comprises: (1) recycling 2-mercaptobenzothiazole, (2) recysling triethylamine, and (3) recycling dichloromethane. The invention relates to a complete set of comprehensive recycling schemeof the ceftriaxone sodium dichloromethane mother liquid. According to the invention, the method provided by the invention has the advantages of simple operation, high yield, low cost, little pollutionand the like; the comprehensive recycling of 2-mercaptobenzothiazole, triethylamine, and dichloromethane can be implemented; the recycling of organic matter is maximized, and organic pollution environment is reduced; waste water generated from a recycling process is applied for reducing the dosage of acid and alkali in an extraction process, and meanwhile, the discharge of waste water is also controlled. The method provided by the invention has a good practical prospect.
Owner:SHANXI WEIQIDA PHARMA IND
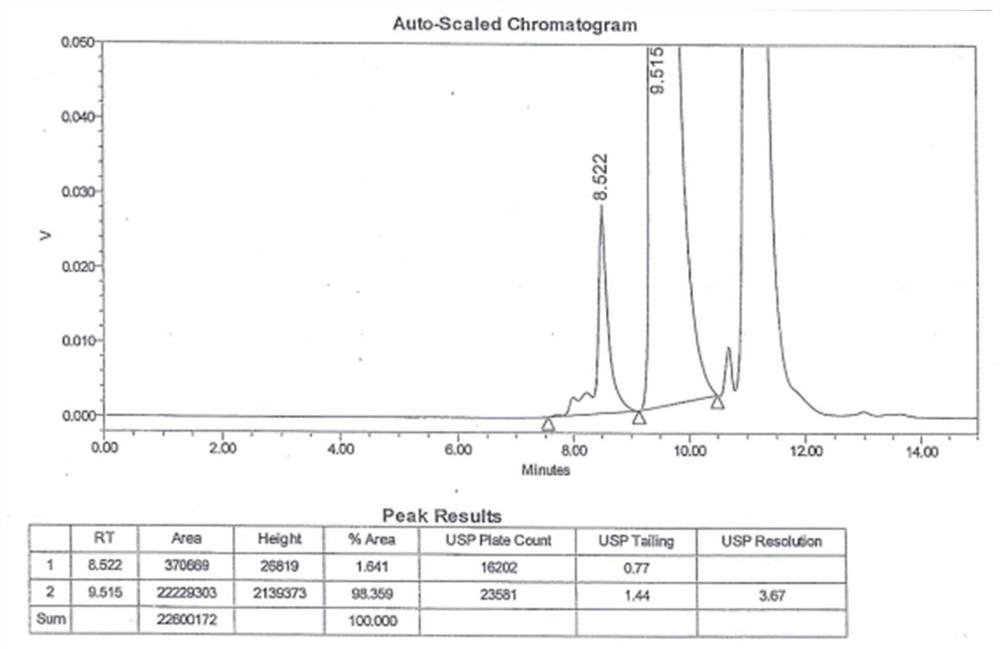
Detection method for antioxidant in ceftriaxone sodium for injection
InactiveCN104198618AAuxiliary control of product qualityAvoid product qualityComponent separationSodium ceftriaxoneAnti oxidant
The invention discloses a detection method for an antioxidant in ceftriaxone sodium for injection. According to the method, the antioxidant in ceftriaxone sodium for injection can be detected in an appropriate detection concentration through the establishment of a linear equation and the determination of precision degree, so that the product quality of the ceftriaxone sodium for injection can be further controlled in an auxiliary mode; the method is practical and simple, and a new direction of controlling the product quality of the ceftriaxone sodium for injection is developed.
Owner:SICHUAN PHARMA
Medicinal composition containing ceftriaxone sodium and lidocaine hydrochloride injection
InactiveCN1943578AProduct quality is stable and controllableReasonable formulaAntibacterial agentsPowder deliveryCeftriaxone SodiumDrug
The invention relates to a medicine composition for injection containing ceftriaxone sodium and Lidocaine Hydrochloride, and characterized by said composition contains 50mg-5000mg ceftriaxone sodium, 1mg-100mg Lidocaine Hydrochloride. The composition in said invention is prepared by split charging of germ-free ceftriaxone sodium and Lidocaine Hydrochloride in accordance with specifications.
Owner:GUANGZHOU PUIS PHARMA FACTORY
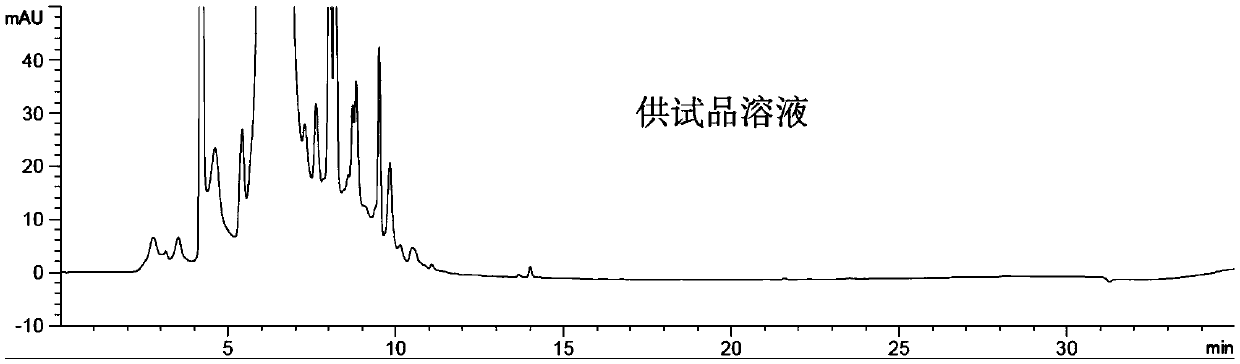
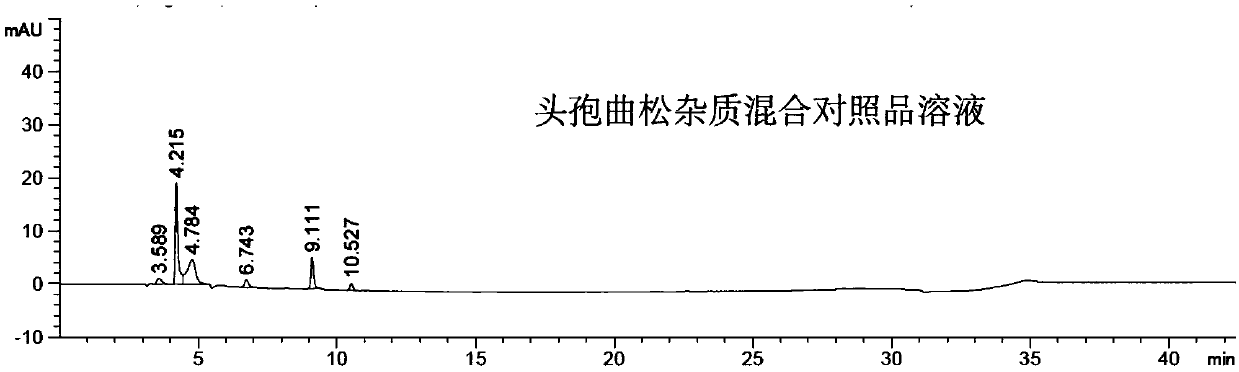
Method for detecting impurity 2-mercaptobenzothiazole in ceftriaxone sodium
ActiveCN111208215AStrong detection specificityGood linear fitComponent separationThiamazolumPhosphate
The invention relates to a method for detecting impurity 2-mercaptobenzothiazole in ceftriaxone sodium by using high performance liquid chromatography. The detection method adopts the following chromatographic conditions: a mobile phase A: a mixed solution of a phosphate buffer solution with the pH value of 6.05-6.45 and methanol; and a mobile phase B: an acetonitrile aqueous solution; wherein themobile phase A and the mobile phase B are used for gradient elution. The method is good in detection specificity, good in linear fitting, low in detection limit, high in sensitivity, good in quantification limit repeatability and good in durability.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
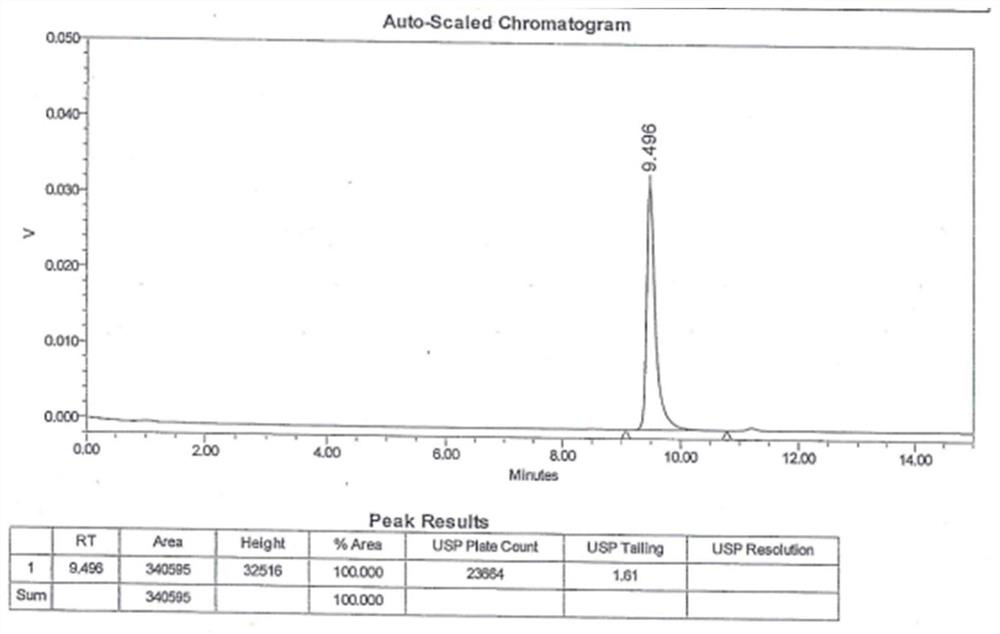
Method for detecting polymer impurities of ceftriaxone sodium
PendingCN112798705AStrong specificityImprove linearityComponent separationO-Phosphoric AcidSilica gel
The invention belongs to the technical field of drug detection, and particularly relates to a method for detecting polymer impurities of ceftriaxone sodium. According to the method, a ceftriaxone sodium raw material medicine is taken to prepare a test solution, HPLC detection is carried out, and the detection conditions are as follows: a chromatographic column with globular protein hydrophilic modified silica gel as a filler is adopted as a stationary phase; and the mobile phase is a mixed solution composed of a mobile phase A and a mobile phase B in a volume ratio of (93-97): (7-3), the mobile phase A is a 0.1-0.2% triethylamine solution, the pH value is adjusted to 5.5-6.5 by using a phosphoric acid solution, and the mobile phase B is acetonitrile. The method for detecting the polymer impurities in the ceftriaxone sodium is high in specificity, good in operability and good in accuracy and precision.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
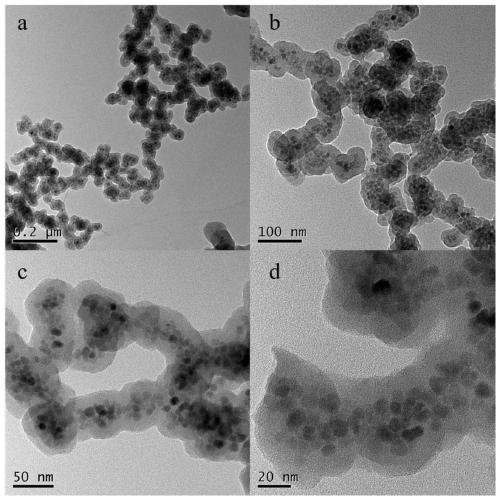
Preparation method of high-selectivity ceftriaxone sodium magnetic molecularly imprinted polymer
ActiveCN111269366ASuperparamagneticImprove adsorption capacityOther chemical processesAlkali metal oxides/hydroxidesPolymer scienceFunctional monomer
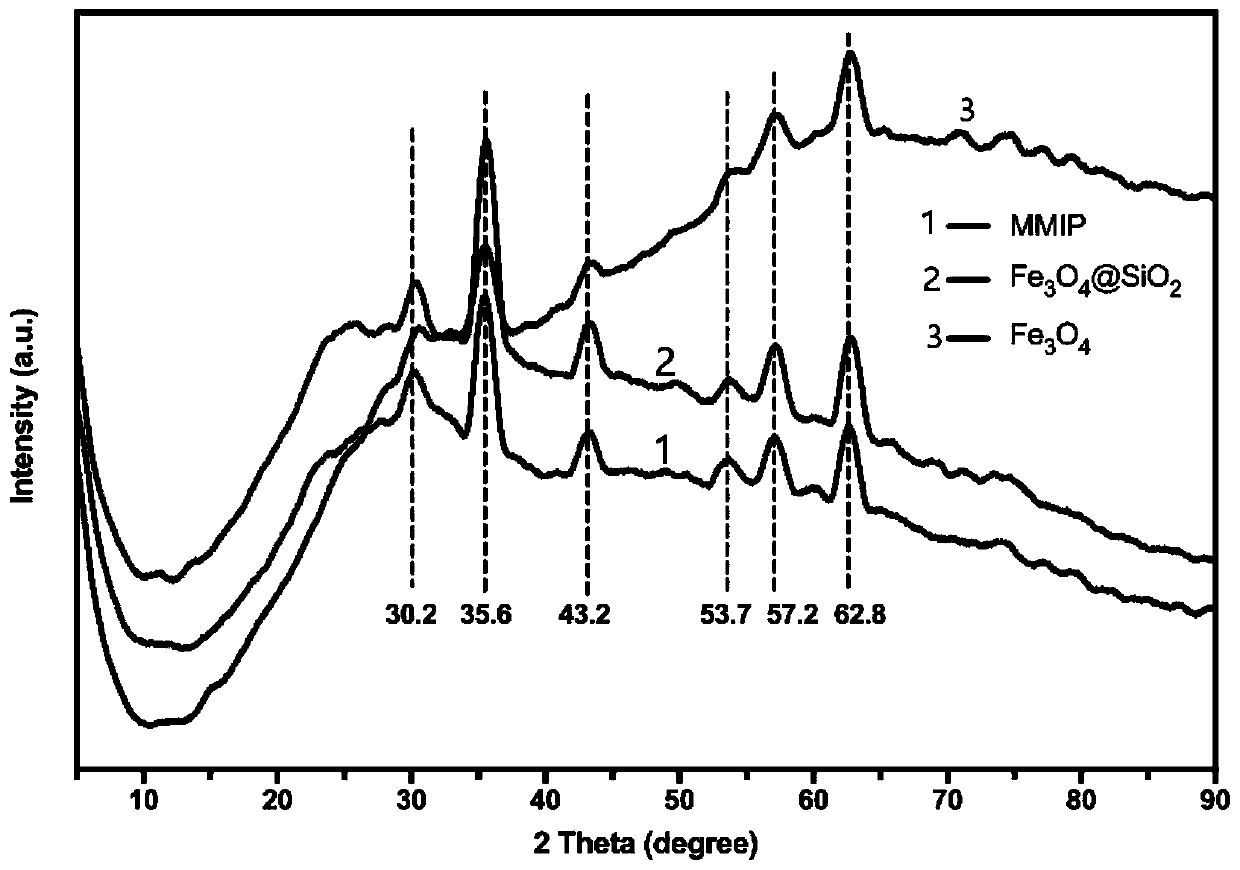
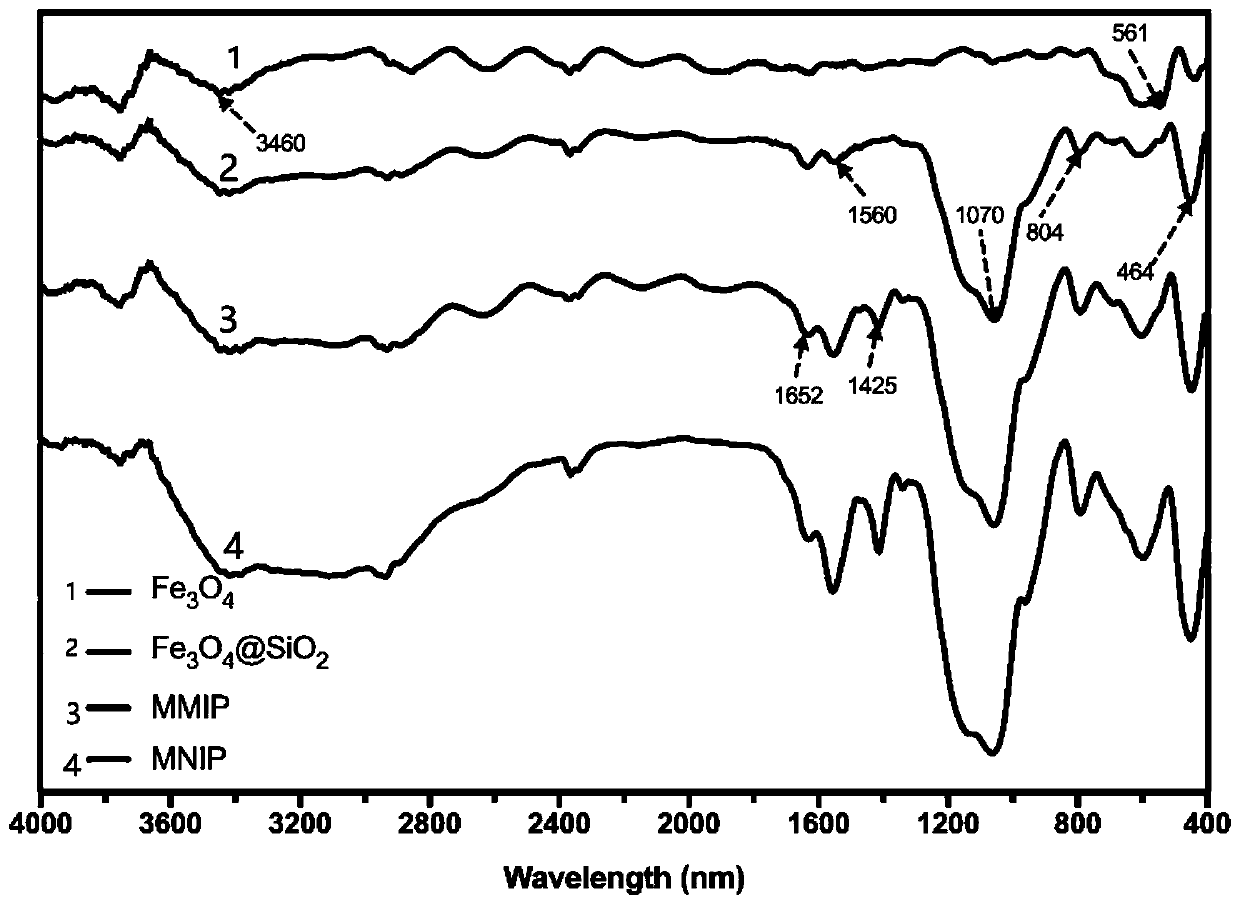
The invention discloses a preparation method of a high-selectivity ceftriaxone sodium magnetic molecularly imprinted polymer. The preparation method comprises the following steps: firstly, synthesizing superparamagnetic Fe3O4 nanoparticles with hydroxylated surfaces in one step by taking triethylene glycol and ferric acetylacetonate as raw materials through a polyol method; then taking the superparamagnetic Fe3O4 nano particles, tetraethoxysilane and ammonia water as raw materials, preparing Fe3O4@SiO2 nano particles by a sol-gel method, and modifying amino groups on the surfaces of the nano particles by utilizing 3-aminopropyltriethoxysilane; then with the ceftriaxone sodium as a template molecule, methacrylic acid as a functional monomer, acetonitrile and methanol as pore-foaming agents,ethylene glycol dimethacrylate as a cross-linking agent and azodiisobutyronitrile as an initiator, preparing the magnetic molecularly imprinted polymer with specific adsorption performance on ceftriaxone sodium. The superparamagnetism and specific adsorption capacity of the ceftriaxone sodium magnetic molecularly imprinted nanoparticles are effectively improved. The preparation method is simple,the cost is low, and the ceftriaxone sodium magnetic molecularly imprinted nanoparticles have wide application prospects.
Owner:HANGZHOU DIANZI UNIV
Preparation method of ceftriaxone sodium spherical crystal
Owner:SHENZHEN CHINA RESOURCES GOSUN PHARMA CO LTD
Ceftriaxone sodium composition freeze-dried powder for injection
InactiveCN103536555AHigh activityShort cycle of medicationAntibacterial agentsOrganic active ingredientsChitosan nanoparticlesSodium ceftriaxone
The invention provides a ceftriaxone sodium composition freeze-dried powder for injection, and belongs to the field of medicine and medicine preparation technology. The ceftriaxone sodium composition freeze-dried powder comprises following raw material ingredients, by weight, 7.26 to 9.17 parts of ceftriaxone sodium, 5.78 to 7.67 parts of chitosan nanoparticle, and 81.38 to 87.10 parts of injection water. Advantages of the ceftriaxone sodium composition freeze-dried powder are that: 1) activity of the ceftriaxone sodium composition in treatment of gonorrhoea is increased significantly, so that combination medication of other antibiotics with ceftriaxone sodium with unknown action mechanisms is avoided in clinic; 2) antibacterial spectrum is widened, and drug tolerance is reduced greatly; 3) improvement of activity is capable of shortening medication cycle of patients, and reducing occurrence likelihood of adverse reaction caused by accumulation of ceftriaxone sodium; and 4) the chitosan nanoparticle can be used as a freeze-dried skeleton agent of the freeze-dried powder injection instead of mannitol, so that active effects of mannitol on human bodies are avoided.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Medicament for treating pulmonitis, and high fever, lower fever non-reduction, and formulating method
InactiveCN101244137AGood curative effectOrganic active ingredientsAntipyreticCurative effectAminocaproic acid
The invention discloses a medicine for treating pneumonia and high / low fever as well as the preparation method, mainly comprising a Qingkailing injection, a ceftriaxone sodium for injection and an aminocaproic acid injection which are formed according to weight proportion. The medicine has the advantages of rapid curative effect and high healing rate in treating pneumonia as well as high / low fever.
Owner:郑明刚
Comprehensive Recovery Method of 2-Mercaptobenzothiazole, Triethylamine and Dichloromethane in Ceftriaxone Sodium Dichloromethane Mother Liquor
ActiveCN109053627BMeet the requirements of production recyclingReduce dosageAmino compound purification/separationHalogenated hydrocarbon preparationCeftriaxoneTriethylamine
Owner:SHANXI WEIQIDA PHARMA IND
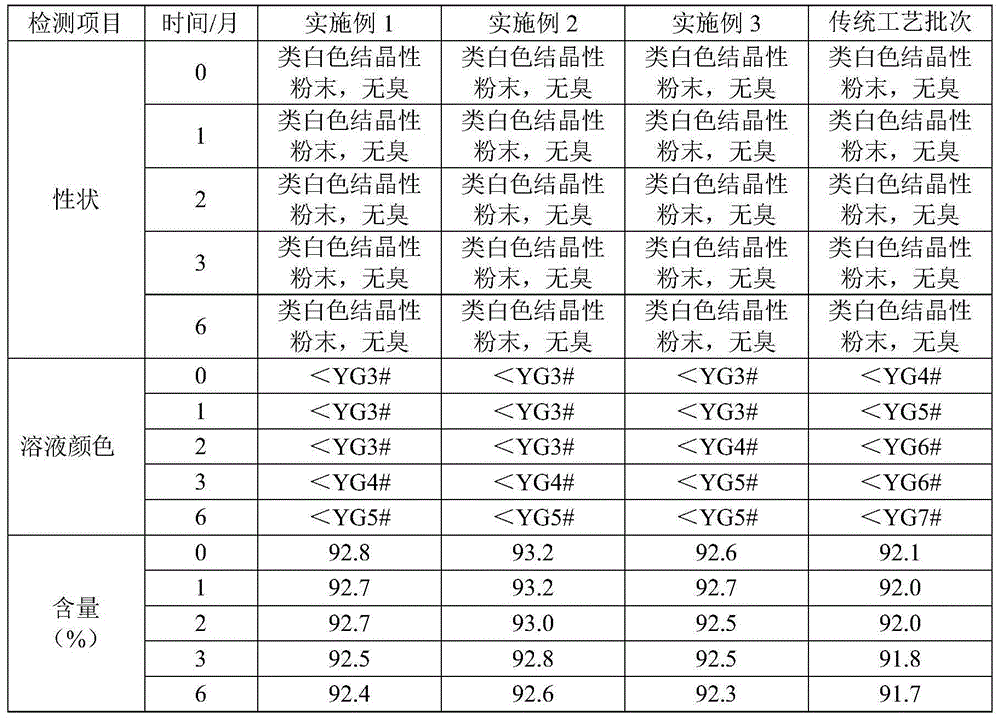
Preparation method of ceftriaxone sodium crystal with good stability and high operability
PendingCN111909180AImprove playbackFacilitate functioningOrganic chemistry methodsActivated carbonOrganic solvent
The invention relates to the technical field of pharmaceutical preparations, in particular to a preparation method of a ceftriaxone sodium crystal with good stability and high operability. The preparation method of the ceftriaxone sodium crystal with good stability and high operability comprises the following steps: firstly, stirring and dissolving an organic solvent, water and ceftriaxone sodiumin a mass ratio of 1-10: 1-5: 1 at the temperature of 5-25DEG C and the stirring rate of 10-45Hz to obtain a seed crystal; dissolving the ceftriaxone sodium crude product into purified water, adding activated carbon after dissolving, and performing stirring, and filtering to obtain filtrate for later use; slowly dropwise adding acetone into the filtrate, adding crystal growth crystals, then continuously adding acetone to obtain a crystallization solution, and sequentially performing cooling, filtering, washing and drying to obtain ceftriaxone sodium crystals. The preparation method is simple in reaction condition, easy to operate, easy to control and good in repeatability, and the obtained ceftriaxone sodium crystal is uniform in particle, good in crystal form, good in stability and suitable for industrial production.
Owner:REYOUNG PHARMA
Preparation method of ceftriaxone sodium
ActiveCN112679524AQuality improvementReduce adverse reactionsOrganic chemistrySodium hydroxideCeftriaxone
The invention discloses a preparation method of ceftriaxone sodium, which comprises the following steps: carrying out earlier-stage treatment on AE active ester and 7-ACT to respectively obtain a first mixed solution, a second mixed solution and a third mixed solution, and simultaneously adding the third mixed solution, the second mixed solution and triethylamine into the first mixed solution to obtain a first reaction solution; adding a sodium hydroxide aqueous solution, sodium metabisulfite and EDTA into the first reaction solution, conducting stirring to obtain a second reaction solution, adding dichloromethane into the second reaction solution, conducting standing for phase splitting to obtain a dichloromethane phase and a water phase, adding activated carbon into the water phase for decoloration, conducting vacuum pumping, and then conducting filtering through a decarburization filter to obtain a to-be-crystallized liquid of ceftriaxone acid; treating the ceftriaxone acid to-be-crystallized liquid to obtain a ceftriaxone acid wet product; then obtaining a to-be-crystallized liquid of ceftriaxone sodium; and adding an acetone solution of methanol into the to-be-crystallized liquid of ceftriaxone sodium for crystallization, and conducting filtering, washing and drying to obtain a finished product of ceftriaxone sodium. According to the invention, high-quality ceftriaxone sodium can be obtained.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
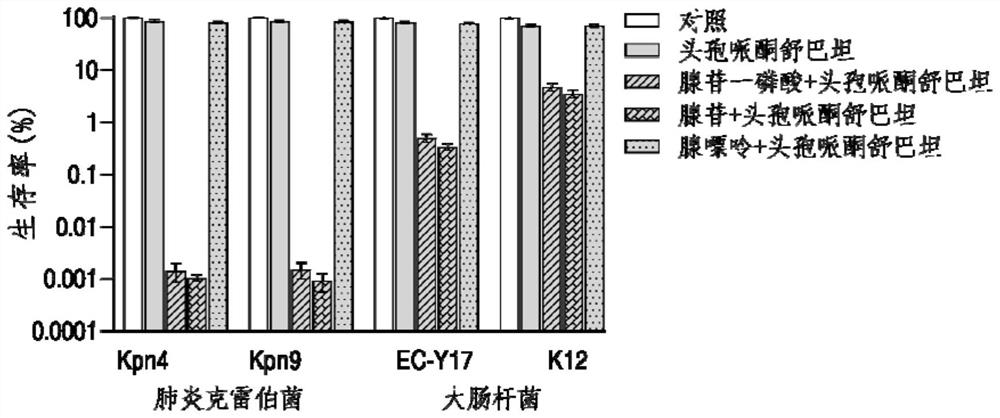
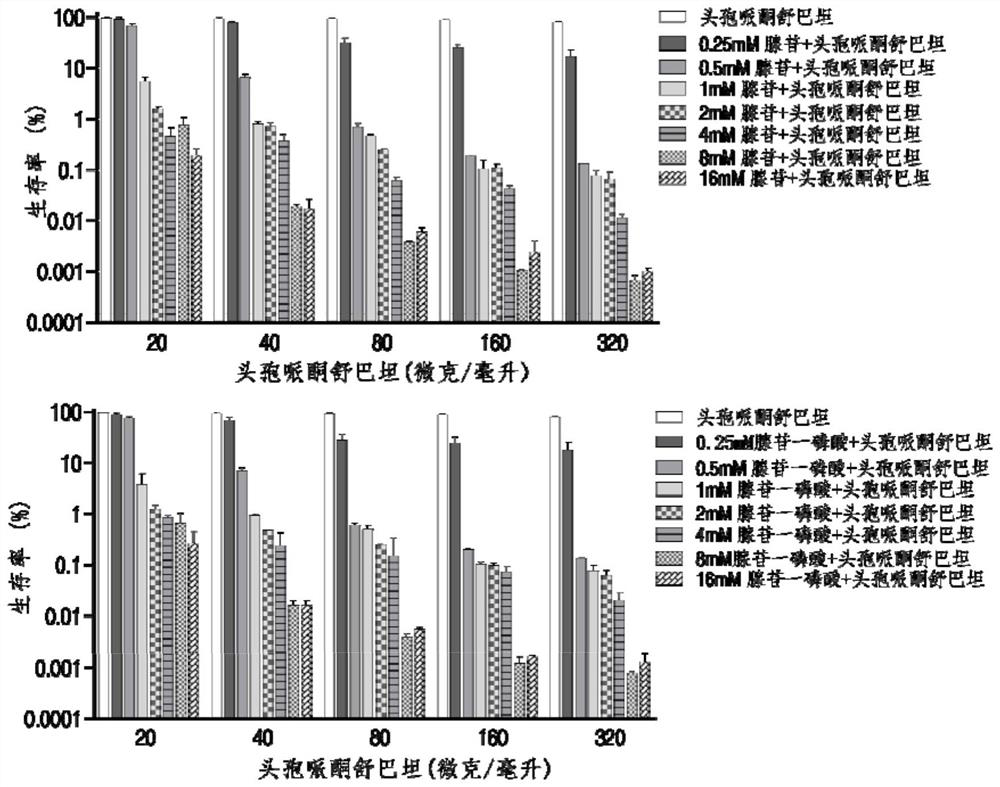
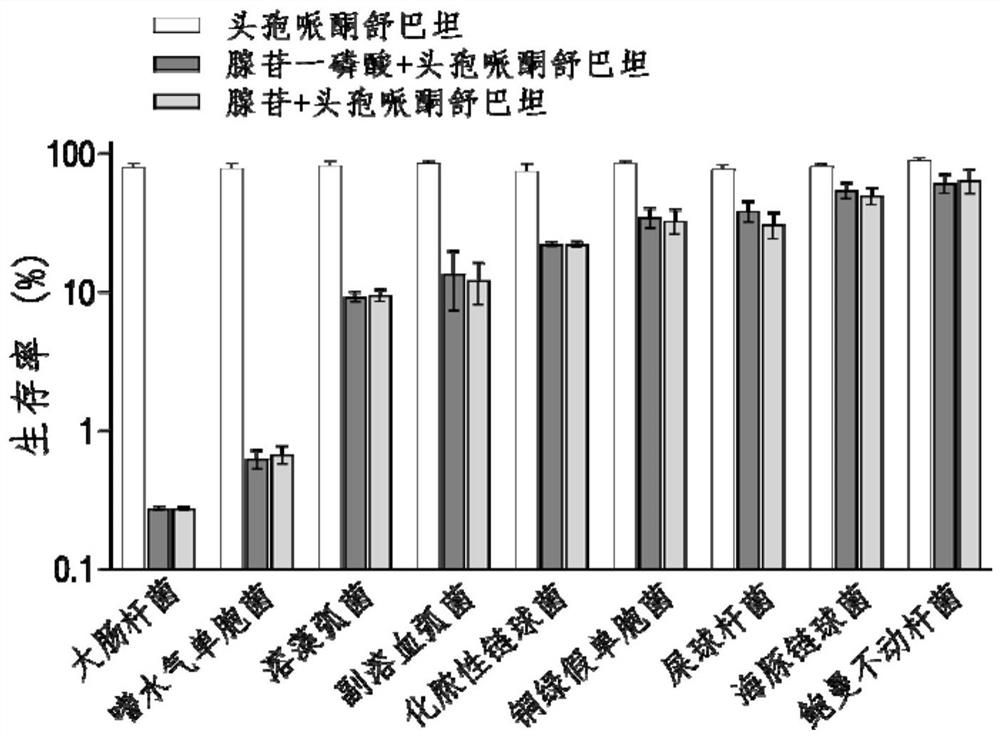
Application of adenosine or adenosine monophosphate in preparation of anti-infective drugs
PendingCN114159456AIncreased sensitivityDelay drug resistanceAntibacterial agentsOrganic active ingredientsMeropenemVibrio parahaemolyticus
The invention belongs to the technical field of biological medicine, and particularly relates to application of adenosine or adenosine monophosphate in preparation of anti-infective drugs. The invention relates to a preparation method of the medicine. Adenosine or adenosine monophosphate can significantly improve the effect of clinical escherichia coli, aeromonas hydrophila, vibrio including vibrio alginolyticus and vibrio parahaemolyticus, streptococcus pyogenes, pseudomonas aeruginosa, bacillus faecium, streptococcus iniae, acinetobacter baumannii, klebsiella pneumoniae and other bacteria on the effects of cefoperazone sulbactam, ceftazidime, ceftriaxone sodium, ceftazidime, ceftriaxone sodium, ceftazidime, ceftriaxone sodium, ceftazidime sodium and the like. The sensitivity of antibiotics such as cefoperazone, meropenem, imipenem, ciprofloxacin, ampenem, moxifloxacin, levofloxacin, gentamicin, amikacin, kanamycin and the like is improved, the compound can be used together with the antibiotics to serve as an anti-infection drug, bacteria are killed under the condition of low-concentration antibiotics, and a good anti-infection effect is achieved; meanwhile, the generation of bacterial drug resistance is reduced.
Owner:SUN YAT SEN UNIV
A kind of ceftriaxone sodium powder injection preparation and preparation method thereof
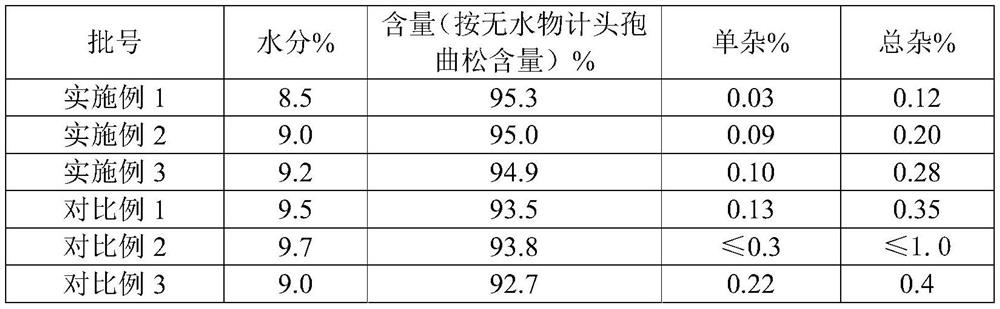
ActiveCN109620804BImprove solubilityImprove product qualityAntibacterial agentsPowder deliveryCeftriaxonumMedicine
The invention relates to a ceftriaxone sodium injection preparation for injection and a preparation method of the ceftriaxone sodium injection preparation, and belongs to the technical field of medicine. The preparation is composed of ceftriaxone sodium and sodium hyaluronate, wherein the sodium hyaluronate is a sodium hyaluronate solution obtained through special treatment of ultrasonic concussion. The sterile ceftriaxone sodium powder for injection is flocculent, the solubleness of the powder injection during fluid preparation is good; the quality of the product is greatly improved, and indexes such as content, single impurity, total impurity and polymer are much higher than the Pharmacopoeia standard and superior to that of the products in the prior art; the stability is significantly improved, through an accelerated test in 6 months, the content is only reduced by 0.1%-0.2%, the single impurity is only reduced by 0.05%-0.06 %, the total impurity is only reduced by 0.10%-0.11%, thepolymer is only reduced by 0.02%, and the stability is obviously superior to that of the products in the prior art.
Owner:石药集团中诺药业(石家庄)有限公司
Chinese and western combined medicine for treating prostatitis
InactiveCN103393842ARegulate immune functionRegulate blood lipidsAntibacterial agentsOrganic active ingredientsHouttuyniaEfficacy
A Chinese and western combined medicine for treating prostatitis is used by combining the following medicines: in parts by mass, 0.8 part of cotrimoxazole tablet, 0.4 part of norfloxacin pulvis, 0.5 part of ceftriaxone sodium injecting pulvis, 10 parts of forsythia suspense, 10 parts of cordate houttuynia, 10 parts of root bark of white mulberry, 9 parts of hippocampus and 9 parts of wolfberry. The Chinese and western combined medicine for treating prostatitis helps to achieve the purpose of treating prostatitis by using western medicine efficacy of strong disinfection and using traditional Chinese medicine efficacies of clearing heat, eliminating inflammation, harmonizing qi and blood, enhancing organism immunity.
Owner:李先强
New Application of Xiaochaihu Granules Combined with Antibiotics
ActiveCN111529671BHas anti-MRSA effectEnhanced inhibitory effectAntibacterial agentsOrganic active ingredientsPharmaceutical SubstancesAntibacterial agent
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
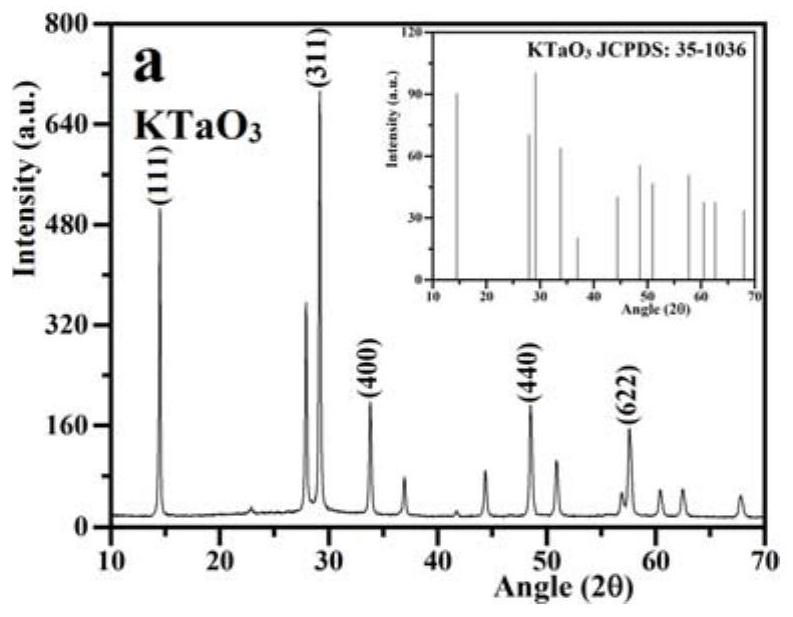
A ternary z-type composite acoustic catalyst for degrading antibiotic wastewater, its preparation method and application
ActiveCN109317157BEasy to separateImprove efficiencyWater contaminantsWater/sewage treatment with mechanical oscillationsPtru catalystSodium ceftriaxone
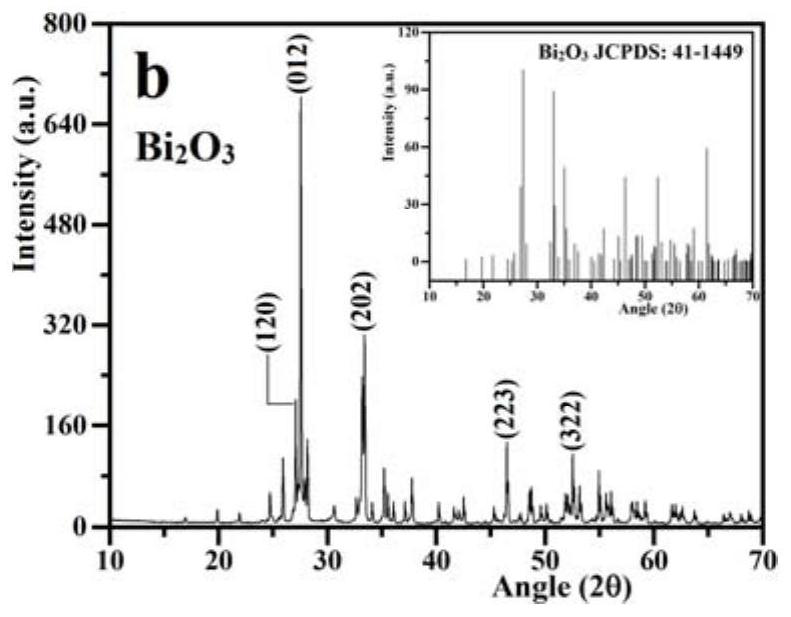
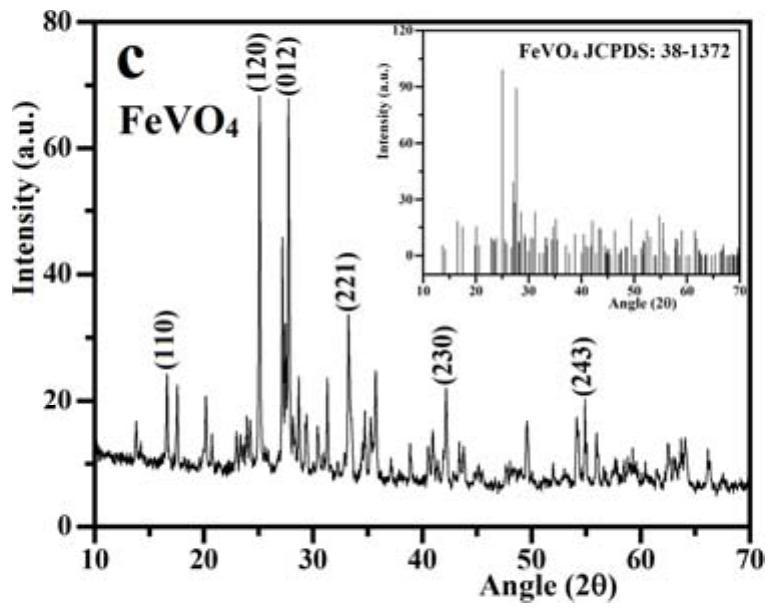
The invention relates to a ternary Z-type composite acoustic catalyst for degrading antibiotic waste water, a preparation method and application thereof. The present invention utilizes hydrothermal method and precipitation method to synthesize ternary Z-type composite acoustic catalyst, in this Z-type acoustic catalyst, KTaO 3 and Bi 2 o 3 Introduce FeVO in the middle 4 The semiconductor forms a redox reaction center, and the combination of the three finally forms a new composite acoustic catalyst KTaO 3 / FeVO 4 / Bi 2 o 3 . Semiconductor FeVO 4 The addition of forms a cyclic redox recombination center, which promotes the Bi 2 o 3 Electrons in the conduction band and KTaO 3 The recombination and suppression of holes on the valence band in KTaO 3 and Bi 2 o 3 Recombination of individual electron-hole pairs. Synthetic Z-type KTaO 3 / FeVO 4 / Bi 2 o 3 The sonocatalyst was applied in the antibiotic wastewater degrading ceftriaxone sodium, and had high sonocatalytic degradation activity.
Owner:LIAONING UNIVERSITY
Wild edible fungus tissue separation method
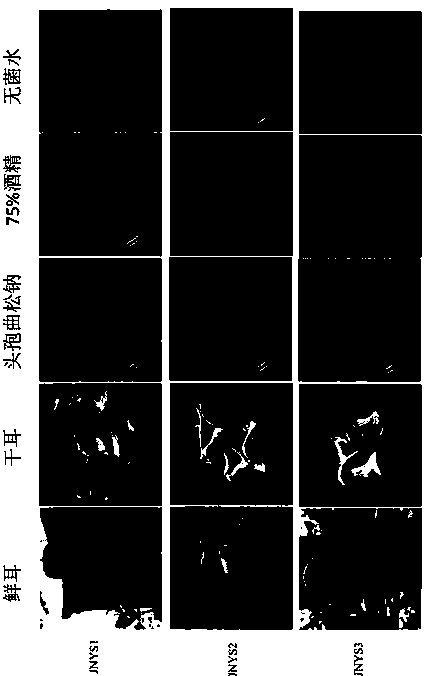
InactiveCN110872564AImprove survival rateShorten the timeFungiMicroorganism based processesBiotechnologySodium ceftriaxone
The invention discloses a wild edible fungus tissue separation method. The method comprises the following steps: 1) collecting edible fungus, removing impurities, and drying the edible fungus at 55-65DEG C to obtain dried edible fungus, (2) taking 0.3-0.6 cm<2> of tissues of the dried edible fungus, and soaking the tissues in a ceftriaxone sodium alcohol solution for 3-5 minutes, and (3) culturing the tissues in a dark place at 22-28 DEG C for 3-4 days, and inoculating a PDA (potato dextrose agar) culture medium with the picked hypha tips. The method has the advantages that 1) compared with low-temperature drying (at 30 DEG C or below) and natural drying, time is saved, the pollution rate caused by a non-laboratory environment is reduced, and a survival rate of collected wild edible fungus resources is increased, 2) only a single antibiotic alcohol solution is used for soaking treatment in the operation process, mercury bichloride and other drugs are not used, and operation is harmless and efficient, 3) according to the tissue separation method, the hypha germination phenomenon can be observed on the third day of inoculation, and the pure strain can be obtained by purification again, and 4) the method is suitable for black fungus, auricularia polytricha and other edible and medicinal macro-fungi with edible and medicinal values which are widely cultivated at present.
Owner:JILIN AGRICULTURAL UNIV
Ceftriaxone sodium and sulbactam sodium composition, pharmaceutical preparation containing composition and application of pharmaceutical preparation
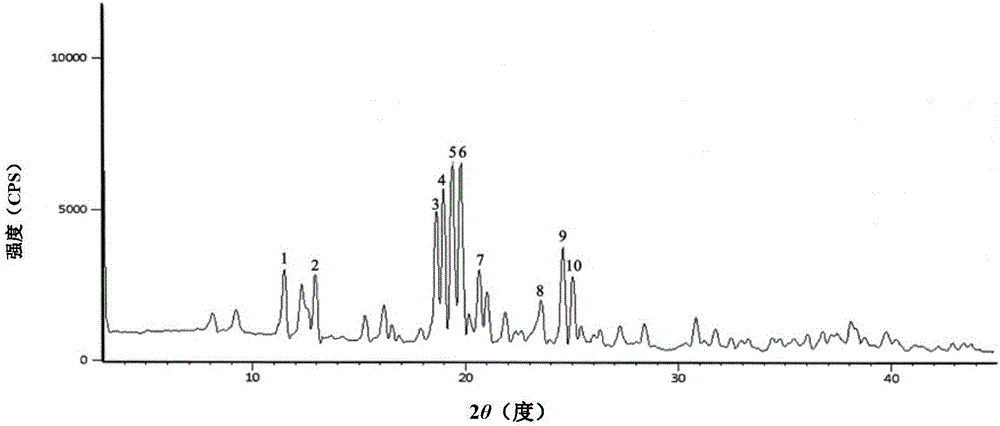
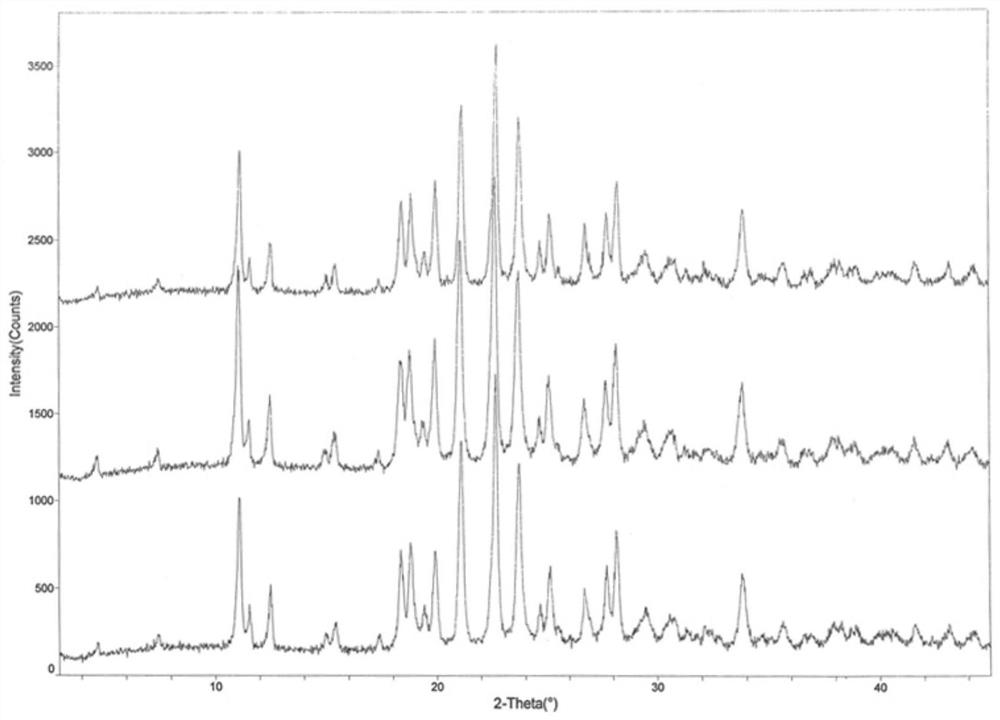
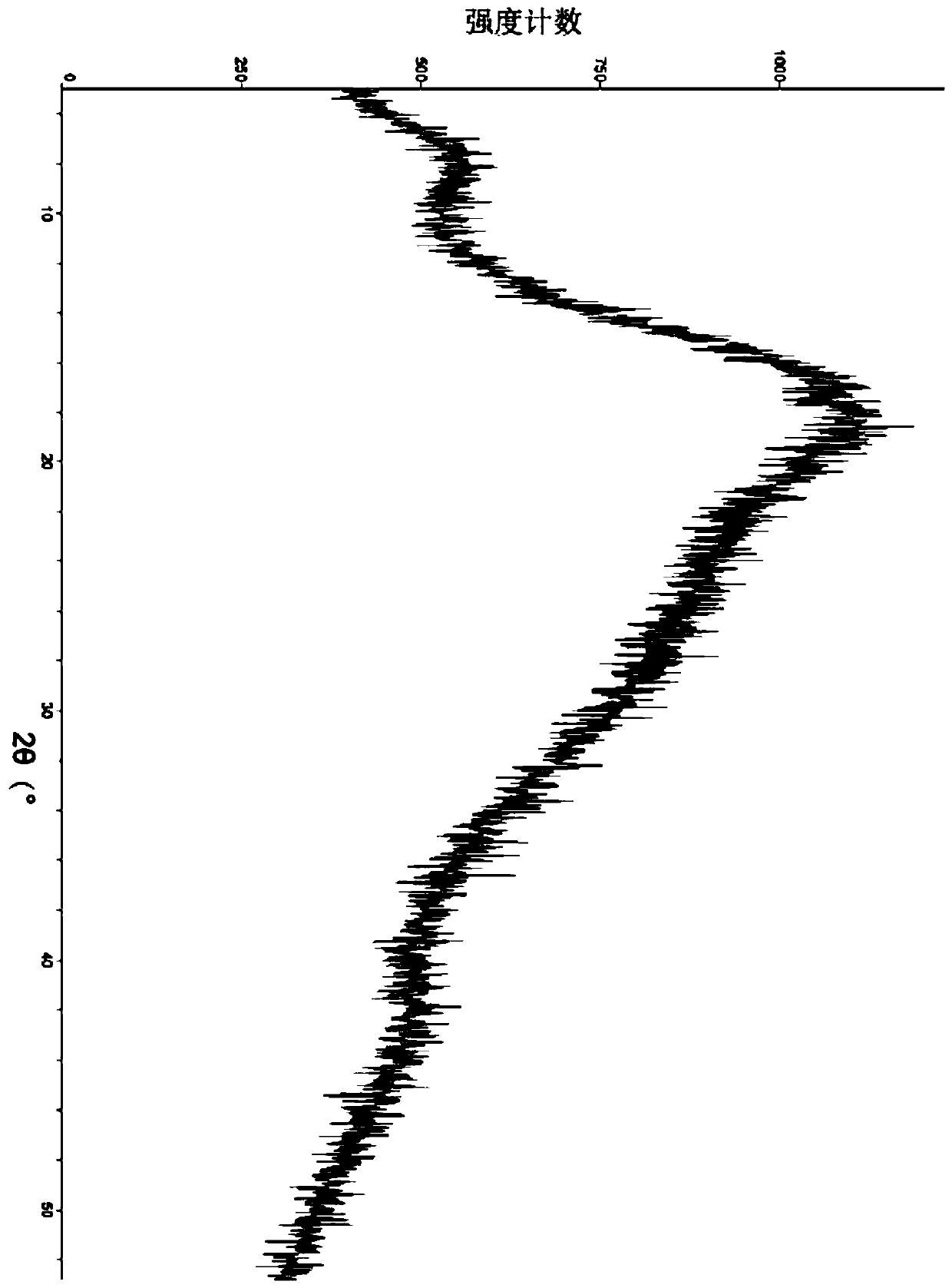
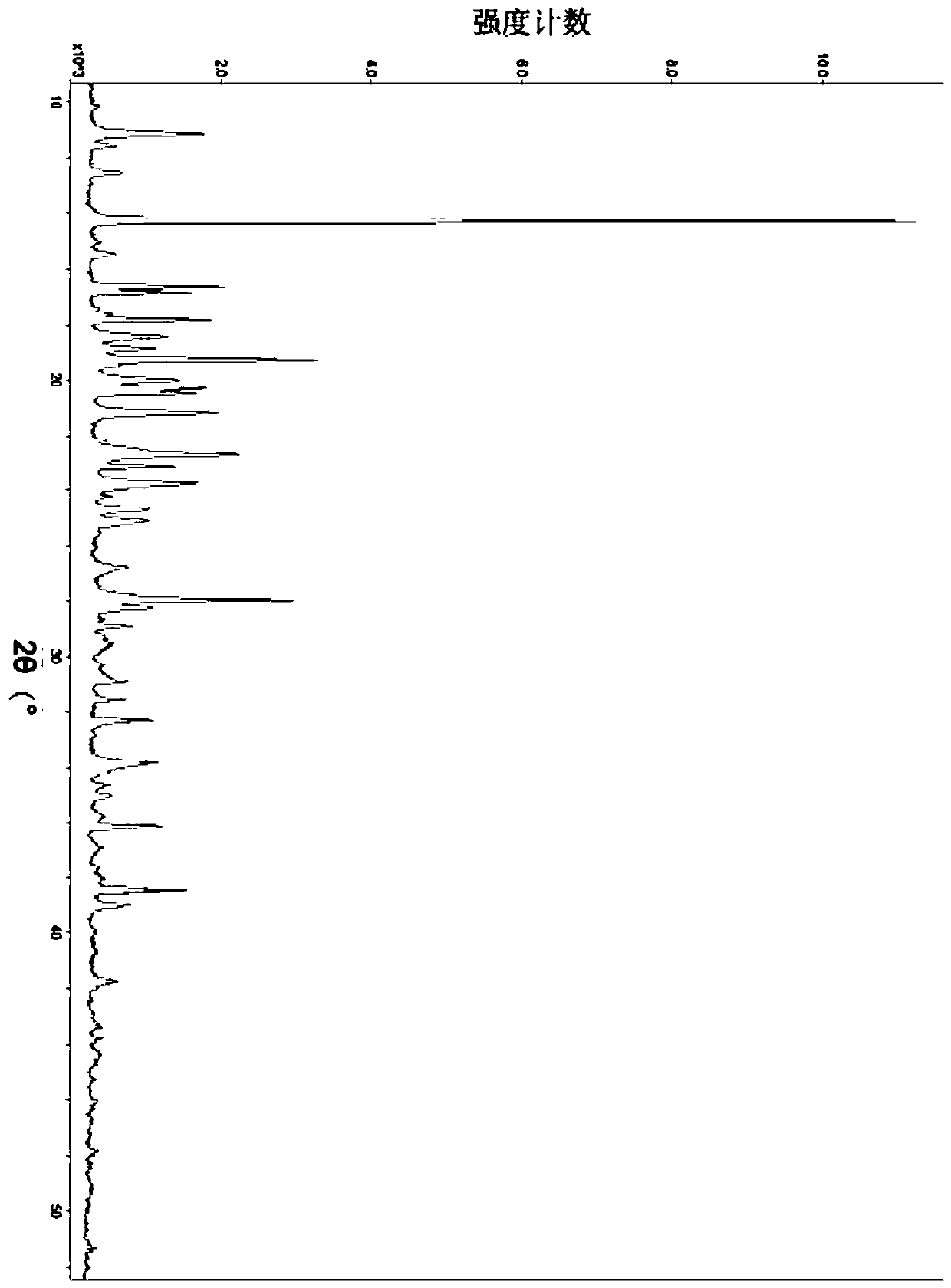
ActiveCN111249284AHigh clarityGood antibacterial effectAntibacterial agentsPowder deliveryDiseaseMacrocyclic lactone
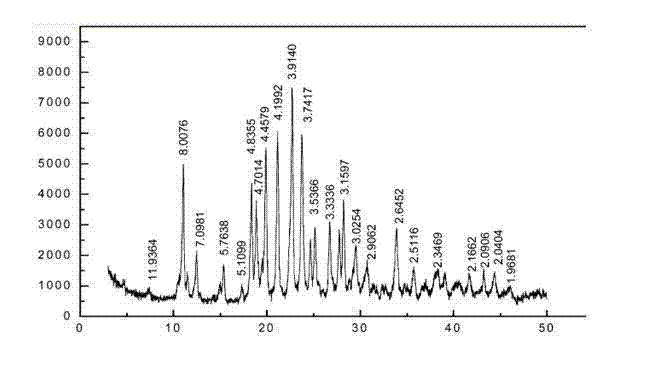
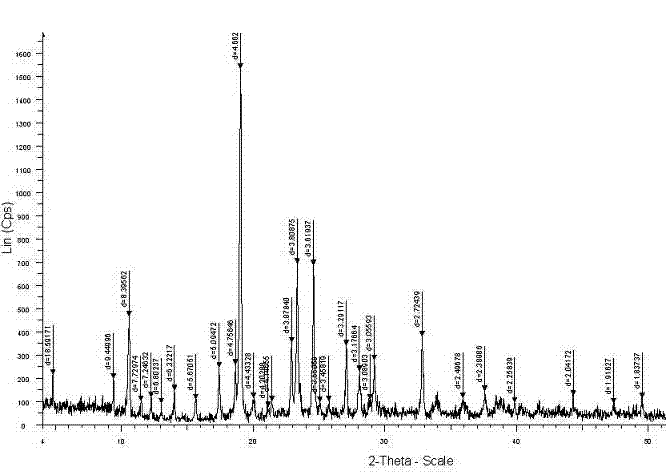
The invention provides a composition composed of ceftriaxone sodium and sulbactam sodium, a pharmaceutical preparation containing the composition and application of the pharmaceutical preparation. Thecomposition is characterized in that an X-ray powder diffraction analysis spectrum contains a specific diffraction angle. The pharmaceutical preparation containing the composition provided by the invention has better bacteriostatic activity and stability; therefore, the preparation is very suitable for treatment of bacterial infection, and is especially suitable for treatment of refractory urogenital system infection diseases caused by Neisseria gonorrhoeae resistant to various antibiotics (beta-lactams, tetracyclines, macrolides, fluoroquinolones or aminoglycosides).
Owner:XIANGBEI WELMAN PHARMA CO LTD +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com