Preparation method of ceftriaxone sodium crystal with good stability and high operability
A ceftriaxone sodium, operable technology, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of poor crystal form and poor stability of ceftriaxone sodium, and achieve good stability, uniform crystal particles, Easy to operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
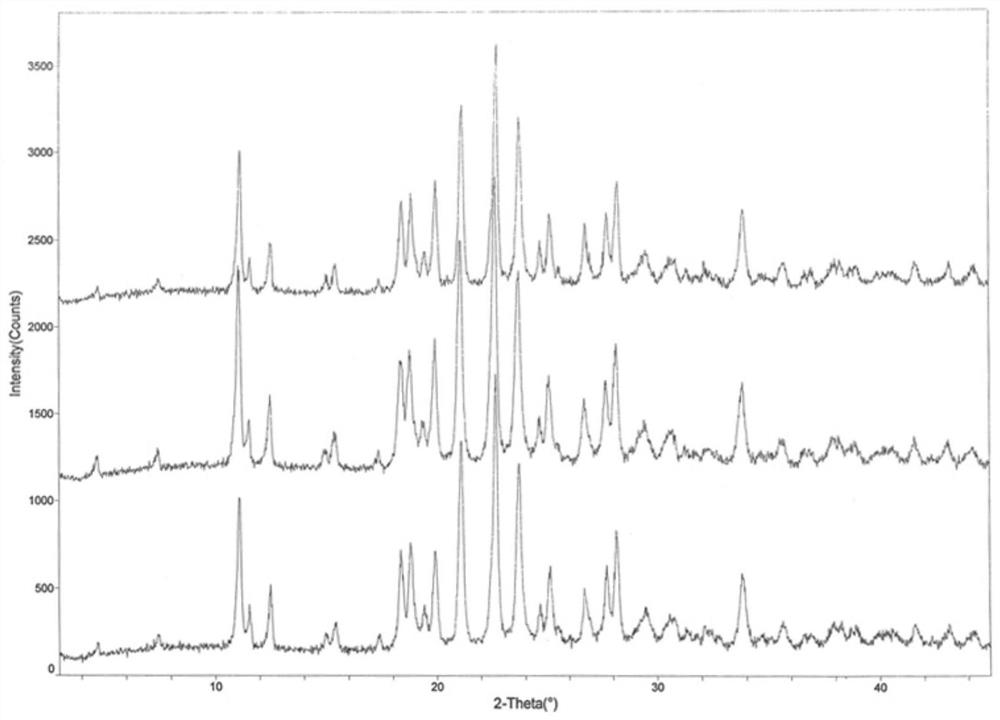
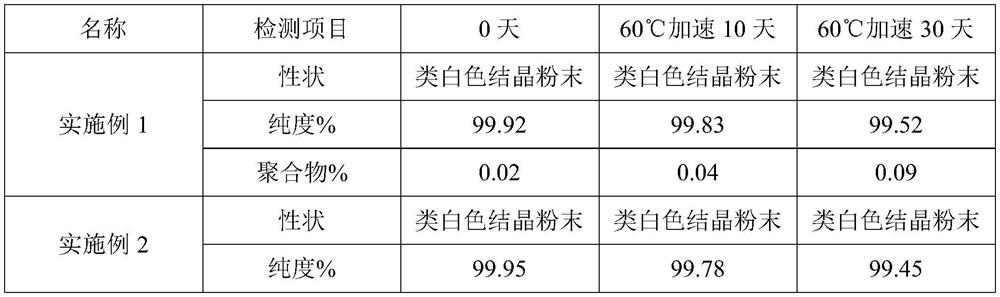
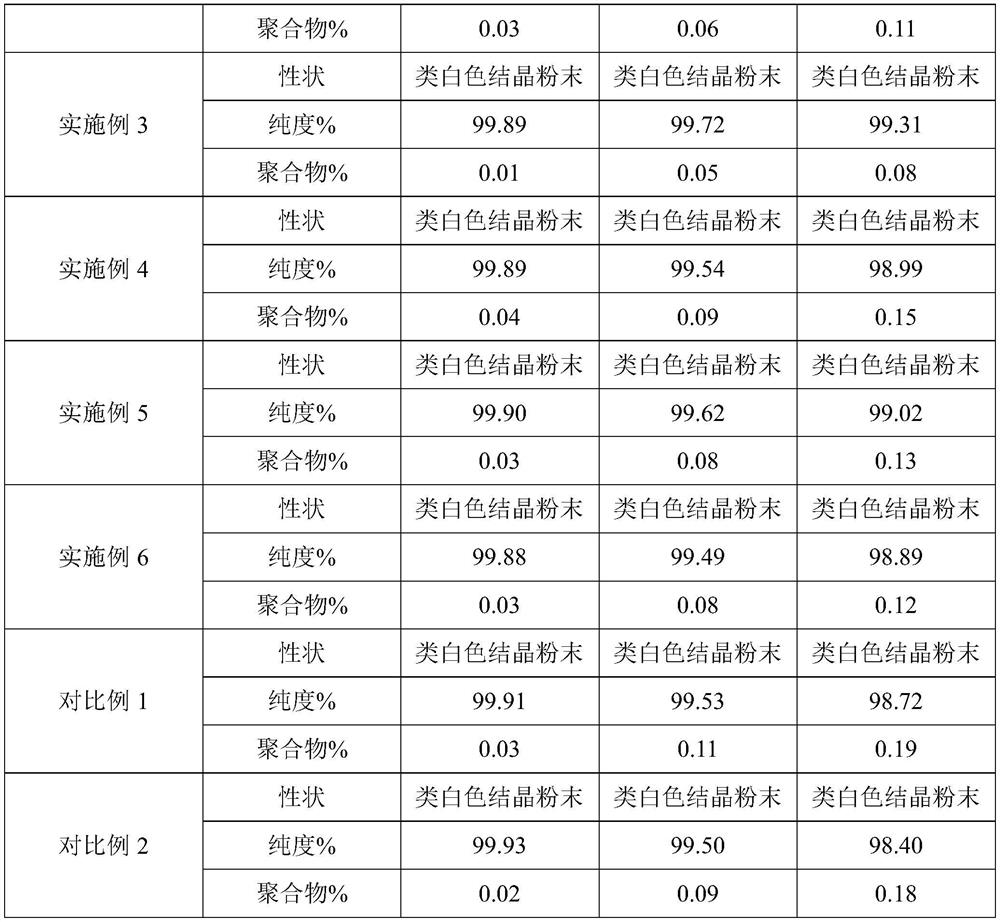
Image
Examples
Embodiment 1
[0030] (1) Weigh 8 g of ethanol, 10 g of water, and 5 g of ceftriaxone sodium, stir and dissolve at a stirring temperature of 15° C. and a stirring rate of 25 Hz as seed crystals;
[0031] (2) Weighing 500 g of the crude product of ceftriaxone sodium and adding it to 1250 g of purified water, stirring and dissolving at 15° C., adding 100 g of activated carbon after dissolving to decolorize and filter with stirring to obtain the filtrate for subsequent use;
[0032] (3) Slowly add 1500g of acetone dropwise to the filtrate obtained in step (2), and no solid precipitates at this time, then add the seed crystal prepared in step (1), maintain 15°C, grow the crystal for 30min, then continue to add 7500g of acetone, and stir Crystallization solution was obtained after 4h;
[0033] (4) The crystallization solution obtained in step (3) is lowered in temperature, filtered, washed, and dried in sequence to obtain ceftriaxone sodium crystals.
Embodiment 2
[0035] (1) Weigh 8 g of acetone, 10 g of water, and 5 g of ceftriaxone sodium, stir and dissolve at a stirring temperature of 15° C. and a stirring rate of 25 Hz as seed crystals;
[0036] (2) Weighing 500 g of the crude product of ceftriaxone sodium and adding it to 1250 g of purified water, stirring and dissolving at 15° C., adding 100 g of activated carbon after dissolving to decolorize and filter with stirring to obtain the filtrate for subsequent use;
[0037] (3) Slowly add 500g of acetone dropwise to the filtrate obtained in step (2), and no solid is precipitated at this time, then add the seed crystal prepared in step (1), maintain 15°C, grow the crystal for 30min, then continue to add 7500g of acetone, and stir Crystallization solution was obtained after 4h;
[0038] (4) The crystallization solution obtained in step (3) is lowered in temperature, filtered, washed, and dried in sequence to obtain ceftriaxone sodium crystals.
Embodiment 3
[0040] (1) Weigh 5.5 g of isopropanol, 10 g of water, and 5 g of ceftriaxone sodium, stir and dissolve at a stirring temperature of 15° C. and a stirring rate of 25 Hz as seed crystals;
[0041] (2) Weighing 500 g of ceftriaxone sodium crude product and adding it to 1250 g of purified water, stirring and dissolving at 15° C., adding 10 g and 100 g of activated carbon after dissolving to decolorize and filter with stirring to obtain the filtrate for subsequent use;
[0042] (3) Slowly add 1500g of acetone dropwise to the filtrate obtained in step (2), and no solid is precipitated at this time, then add the seed crystal prepared in step (1), maintain 15°C, grow the crystal for 30min, then continue to add 7500g of acetone, and stir Crystallization solution was obtained after 4h;
[0043] (4) The crystallization solution obtained in step (3) is lowered in temperature, filtered, washed, and dried in sequence to obtain ceftriaxone sodium crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com