Crystal form of ceftriaxone sodium and preparation method for crystal form
A technology of ceftriaxone sodium and crystal form, which is applied in the field of medicine, can solve problems such as imperfect crystal form research, and achieve the effect of good stability and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Take 10g of crude ceftriaxone sodium, dissolve it into 100mL of purified water, control the temperature at 20-25°C, add 250mL of acetone dropwise under stirring for 1.5h, stop stirring after the addition is complete, let stand at 20-25°C for 10h, and filter with suction , washed with acetone 100mL, drained, and vacuum-dried to obtain 9.0 g of ceftriaxone sodium as a solid, with a yield of 90%, the detected content was 94.3%, and the crystal form was needle-like.
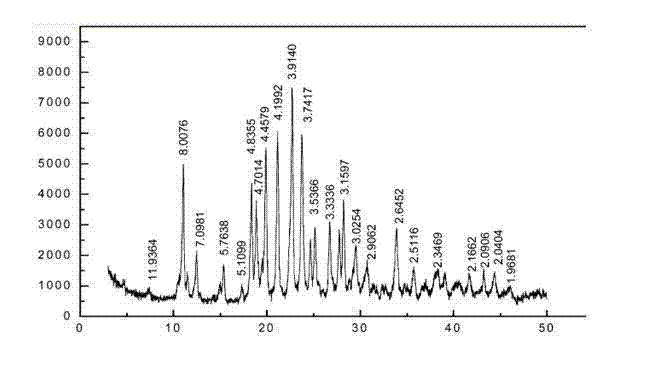
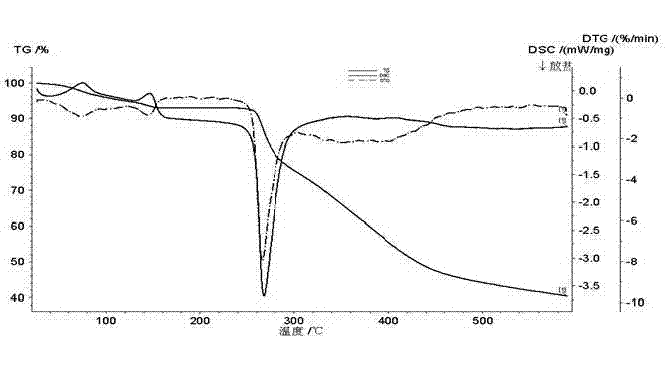
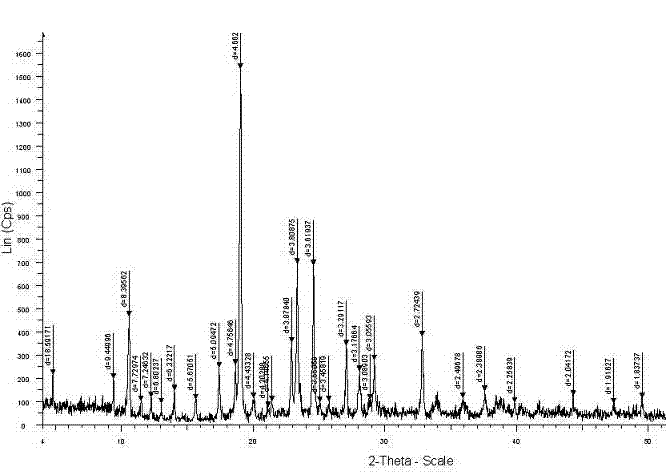
[0052] The resulting product has a melting point of 271°C, and the DSC-DTG-DG spectrum is shown in figure 2 , X-diffraction spectrum data are shown in Table 1, X-diffraction spectrum see image 3 .
[0053] Table 1
[0054] serial number 2θ (°) Peak intensity (%) 1 4.749 13.9 2 9.351 12.8 3 10.529 30.4 4 11.438 6.5 5 12.204 7.6 6 13.004 5.9 7 13.997 9.5 8 15.615 6.9 9 17.392 15.8 10 18.64 16.7 11 19.019 100 12 20.012 7.4 ...
Embodiment 2-5
[0056] Operate according to the method described in Example 1, and the specific parameters are shown in Table 2.
[0057] Table 2
[0058] serial number Crude ceftriaxone sodium (g) Purified water (mL) Add acetone dropwise (mL) Dropping time (h) Standing time (h) Standing temperature (°C) Yield (%) content(%) crystal form Example 2 10 100 260 2 8 20-25 85.6 94.2 Same as Example 1 Example 3 9 100 270 0.5 12 20-25 87.4 94.3 Same as Example 1 Example 4 8 80 280 1.5 10 5-10 84.6 94.2 Same as Example 1 Example 5 5 80 310 2 12 5-10 86.3 94.0 Same as Example 1
Embodiment 6
[0060] Take 32g of crude ceftriaxone sodium, dissolve it into 100mL of purified water, control the temperature at 20-25°C, add 100mL of acetone dropwise under stirring for 1h, stop stirring after the addition is complete, let stand at 5-10°C for 12h, and filter with suction. Wash with 200 mL of acetone, pump dry, and dry in vacuo to obtain 28.7 g of ceftriaxone sodium as a solid, with a yield of 89.6%. The detected content is 94.2%, and the crystal form is flake.
[0061] The melting point of the obtained product is 272°C, and the DSC-DTG-DG spectrum is shown in Figure 4 , the X-diffraction pattern data are shown in Table 3, and the X-diffraction pattern is shown in Figure 5 .
[0062] table 3
[0063] serial number 2θ (°) Peak intensity (%) 1 4.829 20.9 2 7.538 7.2 3 11.153 56.1 4 11.599 7.4 5 12.547 11.3 6 15.053 6.1 7 15.462 22.3 8 17.424 6.4 9 18.408 33.4 10 19.169 33.6 11 19.989 100 12...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com