A kind of ceftriaxone sodium powder injection preparation and preparation method thereof
A technology for ceftriaxone sodium and injection, which is applied in the field of medicine, can solve problems such as slow dissolution rate, instability, and content reduction, and achieve the effects of product quality improvement, stability improvement, and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
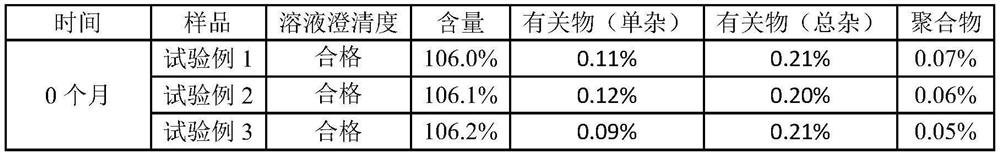
[0051] Embodiment 1 prepares ceftriaxone sodium powder injection preparation for injection (0.25g / bottle) of the present invention
[0052] Preparation:
[0053] Step 1: Take 40g of sodium hyaluronate, dissolve it in 6L of distilled water with slow stirring, perform ultrasonic oscillation at 20 kHz for 30 minutes, and 30 kHz for 1 hour, and cool to room temperature to obtain a sodium hyaluronate solution ,spare;
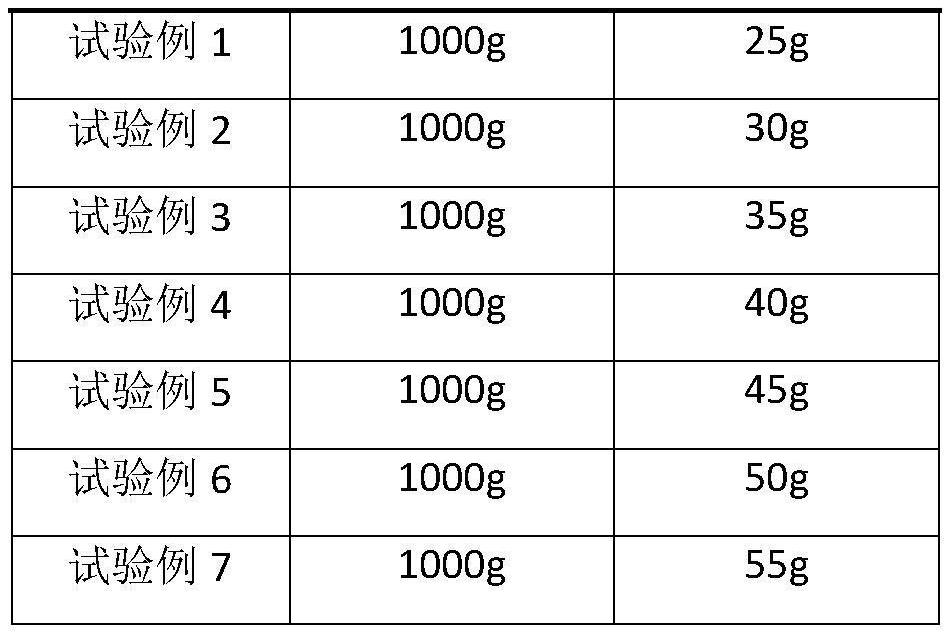
[0054] Step 2: Weigh 1000 g of crude ceftriaxone sodium raw material, add 2 L of distilled water, stir to dissolve, add 4-6% activated carbon to depyrogenate and impurity, stir for 30 minutes, and filter through a microporous membrane to obtain a filtrate;
[0055] Step 3: Add 20% of the sodium hyaluronate solution prepared in step 1 to the filtrate obtained in step 2, stir slowly at 30 rpm for 15 minutes, then add 38% of the sodium hyaluronate solution prepared in step 1, 40 rpm After stirring slowly for 35 minutes, add 26% of the sodium hyaluronate solution prepa...
Embodiment 2
[0059] Embodiment 2 prepares ceftriaxone sodium powder injection preparation for injection (0.5g / bottle) of the present invention
[0060] Preparation:
[0061] Step 1: Take 35g of sodium hyaluronate, dissolve it in 6L of distilled water with slow stirring, perform ultrasonic oscillation at 20 kHz for 30 minutes, and 30 kHz for 1 hour, and cool to room temperature to obtain a sodium hyaluronate solution ,spare;
[0062] Step 2: Weigh 1000 g of crude ceftriaxone sodium raw material, add 2 L of distilled water, stir to dissolve, add 4-6% activated carbon to depyrogenate and impurity, stir for 30 minutes, and filter through a microporous membrane to obtain a filtrate;
[0063] Step 3: Add 20% of the sodium hyaluronate solution prepared in step 1 to the filtrate obtained in step 2, stir slowly at 30 rpm for 15 minutes, then add 38% of the sodium hyaluronate solution prepared in step 1, 40 rpm After stirring slowly for 35 minutes, add 26% of the sodium hyaluronate solution prepar...
Embodiment 3
[0067] Embodiment 3 prepares ceftriaxone sodium powder injection preparation for injection (1.0g / bottle) of the present invention
[0068] Preparation:
[0069] Step 1: Take 45g of sodium hyaluronate, dissolve it in 6L of distilled water with slow stirring, perform ultrasonic oscillation at 20 kHz for 30 minutes, and 30 kHz for 1 hour, and cool to room temperature to obtain sodium hyaluronate solution ,spare;
[0070] Step 2: Weigh 1000 g of crude ceftriaxone sodium raw material, add 2 L of distilled water, stir to dissolve, add 4-6% activated carbon to depyrogenate and impurity, stir for 30 minutes, and filter through a microporous membrane to obtain a filtrate;
[0071] Step 3: Add 20% of the sodium hyaluronate solution prepared in step 1 to the filtrate obtained in step 2, stir slowly at 30 rpm for 15 minutes, then add 38% of the sodium hyaluronate solution prepared in step 1, 40 rpm After stirring slowly for 35 minutes, add 26% of the sodium hyaluronate solution prepared...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com