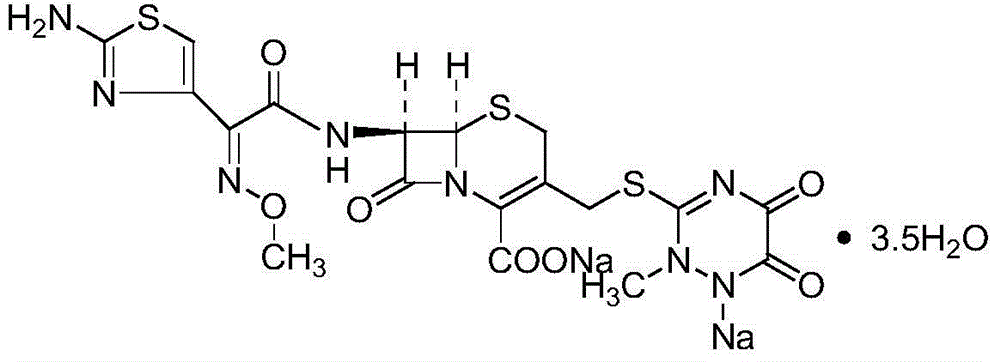
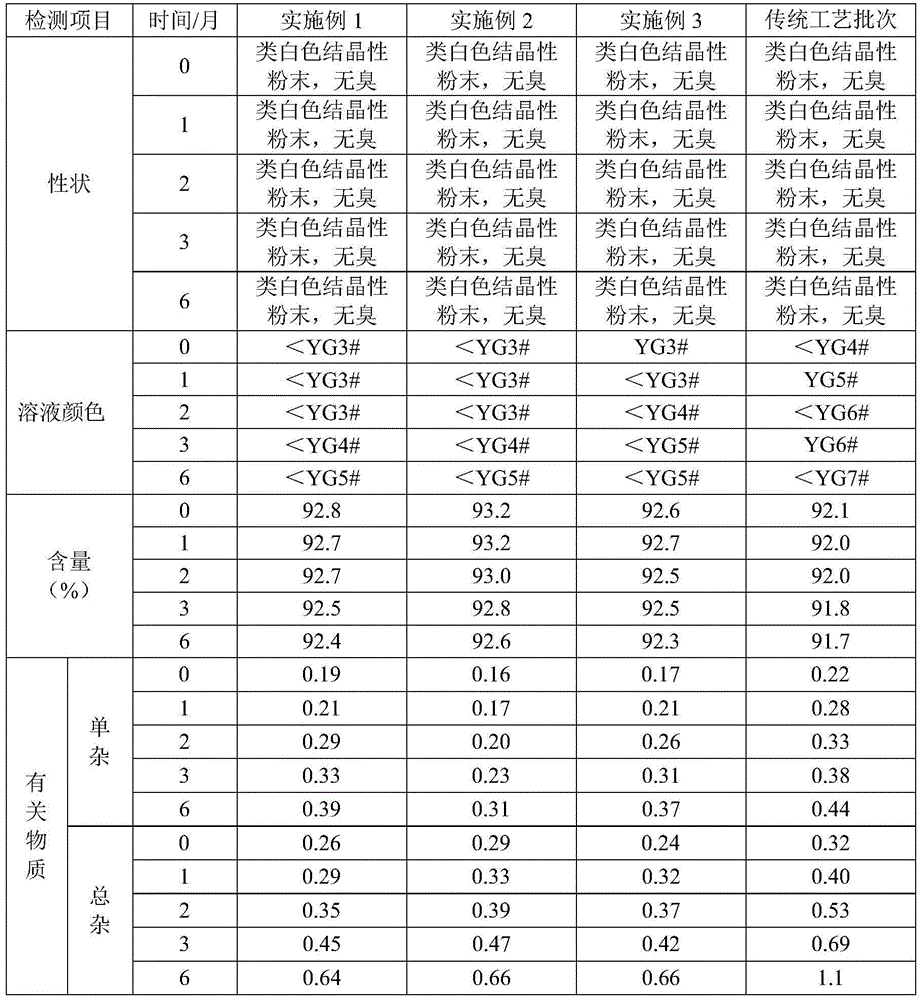
Ceftriaxone sodium powder-injection for injection
A technology for ceftriaxone sodium and injection, which is applied in the field of medicine, can solve problems such as instability, inclusions, and residual solvents, and achieve the effects of high stability, less impurities, and less residual solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Accurately weigh 30g of crude ceftriaxone sodium at 20℃, add 50mL of distilled water, start stirring, and completely dissolve in about 10-15 minutes, then add 3g of activated carbon, decolorize for 30 minutes, filter, and use 60ml volume The mixed solvent with a ratio of (acetone:water=3:1) washes the filter residue and the filter bottle twice, and the filtrate enters the crystallization bottle.
[0029] (2) Add the eluent acetone at a stirring speed of 300 revolutions / min at 15℃:
[0030] Time (min)
[0031] (3) After the crystallization is completed, perform suction filtration, wash the filter cake with 20ml*3 times of acetone, put the filter cake in a vacuum drying oven, the temperature of the vacuum drying oven is 40 ℃, vacuum drying, weighing, and packaging.
Embodiment 2
[0033] (1) Accurately weigh 30g of crude ceftriaxone sodium at 20℃, add 50mL of distilled water, start stirring, and completely dissolve in about 10-15 minutes, then add 3g of activated carbon, decolorize for 30 minutes, filter, and use 60ml The mixed solvent with a ratio of (acetone: water = 2:1) washes the carbon and the filter flask twice, and the filtrate enters the crystallization flask.
[0034] (2) Add the eluent acetone at a stirring speed of 300 revolutions / min at 15℃:
[0035] Time (min)
[0036] (3) Filter, wash and dry. After the crystallization is completed, perform suction filtration. Wash the filter cake with 20ml*3 times of acetone, and put the filter cake in a vacuum drying oven. The temperature of the vacuum drying oven is 35℃, vacuum drying, weighing, and packaging .
Embodiment 3
[0038] (1) Accurately weigh 30g of crude ceftriaxone sodium at 20℃, add 50mL of distilled water, start stirring, and completely dissolve in about 10-15 minutes, then add 3g of activated carbon, decolorize for 30 minutes, filter, and use 60ml volume The mixed solvent with a ratio of (acetone:water=2:1) washes the filter residue and the filter bottle twice, and the filtrate enters the crystallization bottle.
[0039] (2) Add the eluent acetone at a stirring speed of 300 revolutions / min at 15℃:
[0040] Time (min)
[0041] (3) After the crystallization is completed, perform suction filtration, wash the filter cake with 20ml*3 times of acetone, and put the filter cake in a vacuum drying oven with a temperature of 35°C, vacuum drying, weighing, and packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com