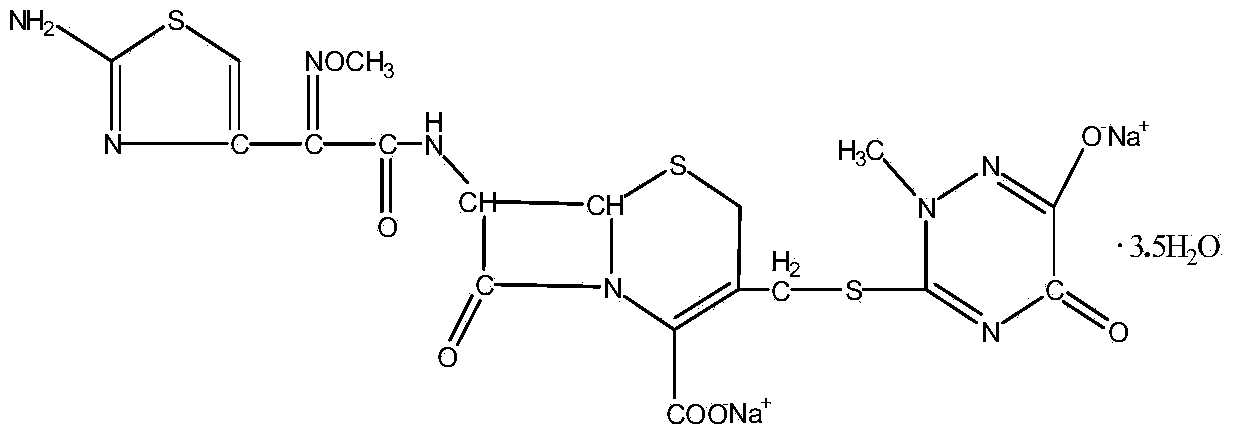
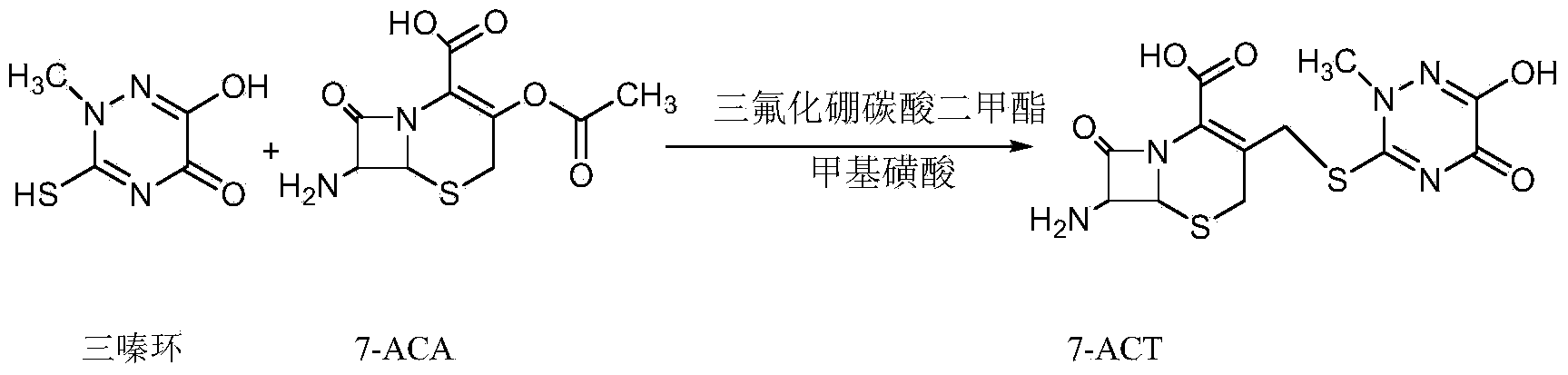
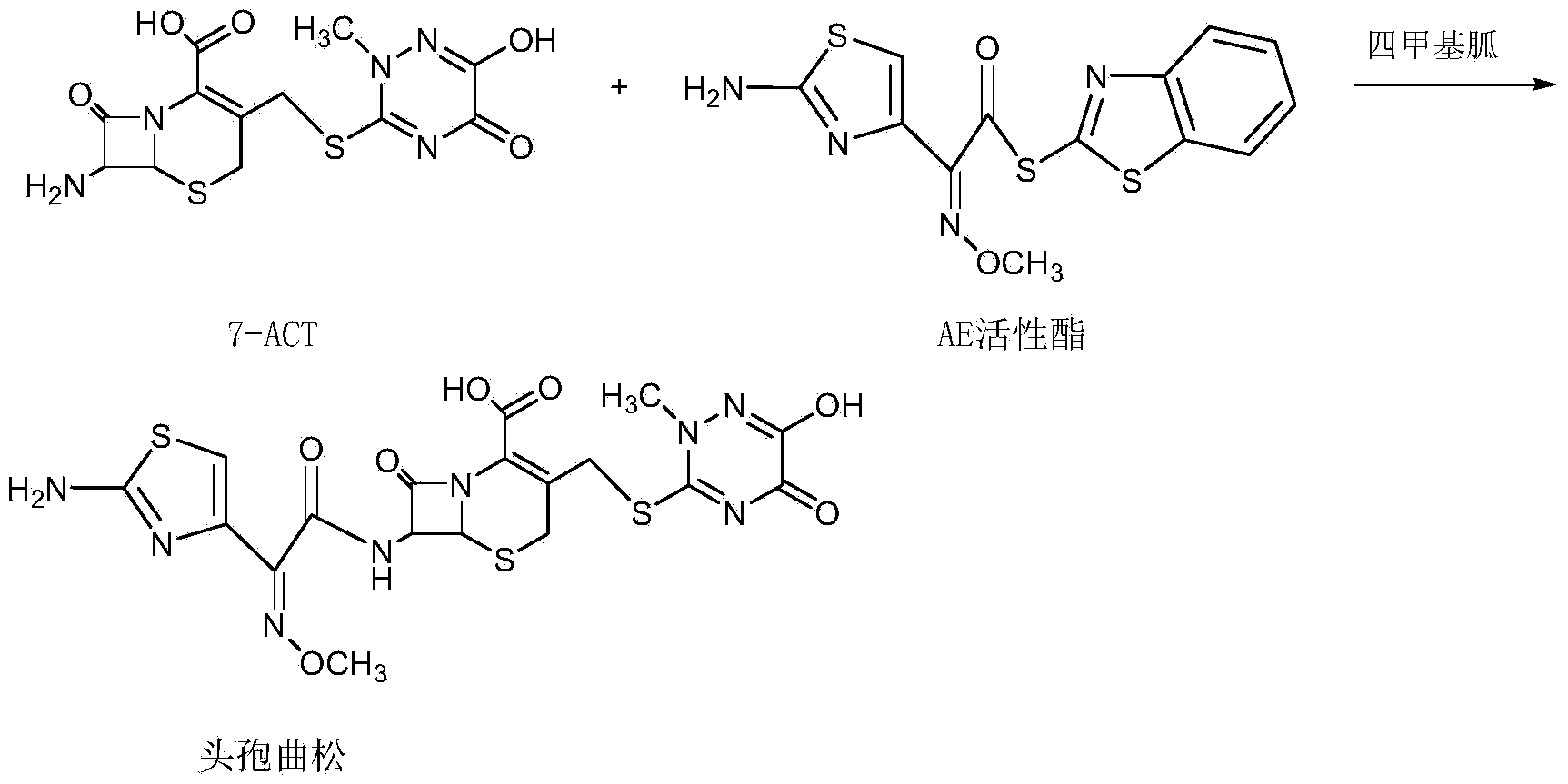
Method for synthesizing ceftriaxone sodium
A technology of ceftriaxone sodium and a synthesis method, which is applied in the field of synthesis of ceftriaxone sodium, can solve the problems such as the influence of 7-ACT on purity and yield, high toxicity of triethylamine, troublesome operation and the like, and improves yield and quality , the effect of reducing the complexity and speeding up the response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of 7-ACT
[0037] Add 120g of dimethyl carbonate, 56g of boron trifluoride dimethyl carbonate, and 8g of methanesulfonic acid into the dry four-necked bottle, stir rapidly and cool down to 10°C, add 15g of triazine ring, 25g of 7-ACA, and keep at 10-12°C The reaction was about 23 minutes, and the sample was detected by HPLC (high performance liquid chromatography) to detect that the residual 7-ACA was less than 1%. The reaction was completed. Add 240g of cold water to the four-necked bottle, the solution is clarified, add 0.25g of EDTA-2Na, 1.0g of sodium dithionite to keep the temperature below 13°C, add diluted ammonia water (1:1) dropwise to adjust the pH = 2.3 to 2.6, and a white solid is precipitated. After dripping, grow the crystal at 5-10°C for 1 hour, filter, wash the filter cake twice with 120 g of water, filter, wash the filter cake once with 50 g of ethanol, filter, and vacuum dry at 45°C for 8 hours to obtain 32.5 g of solid, with a mo...
Embodiment 2
[0038] Example 2 Preparation of 7-ACT
[0039] Add 120g of dimethyl carbonate, 56g of boron trifluoride dimethyl carbonate, and 7g of methanesulfonic acid into the dry four-necked bottle, stir quickly and cool down to 10°C, add 15g of triazine ring, 25g of 7-ACA, and keep at 10-12°C The reaction was about 23 minutes, and the sample was detected by HPLC (high performance liquid chromatography) to detect that the residual 7-ACA was less than 1%. The reaction was completed. Add 240g of cold water to the four-necked bottle, the solution is clarified, add 0.25g of EDTA-2Na, 1.0g of sodium dithionite to keep the temperature below 13°C, add diluted ammonia water (1:1) dropwise to adjust the pH = 2.3 to 2.6, and a white solid is precipitated. After dripping, grow the crystal at 5-10°C for 1 hour, filter, wash the filter cake twice with 120 g of water, filter, wash the filter cake once with 50 g of ethanol, filter, and vacuum dry at 45°C for 8 hours to obtain 32.1 g of solid, with a mo...
Embodiment 3
[0040] Example 3 Preparation of 7-ACT
[0041] Add 120g of dimethyl carbonate, 56g of boron trifluoride dimethyl carbonate, and 6g of methanesulfonic acid into the dry four-necked bottle, stir quickly and cool down to 11°C, add 15g of triazine ring, 25g of 7-ACA, and keep at 10-12°C The reaction was about 25 minutes, and the sample was detected by HPLC (high performance liquid chromatography) to detect that the 7-ACA residue was less than 1%. The reaction was completed. Add 240g of cold water to the four-necked bottle, the solution is clarified, add 0.25g of EDTA-2Na, 1.0g of sodium dithionite to keep the temperature below 13°C, add diluted ammonia water (1:1) dropwise to adjust the pH = 2.3 to 2.6, and a white solid is precipitated. After dripping, grow the crystal at 5-10°C for 1 hour, filter, wash the filter cake twice with 120 g of water, filter, wash the filter cake once with 50 g of ethanol, filter, and vacuum dry at 45°C for 8 hours to obtain 32.0 g of solid, with a mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com