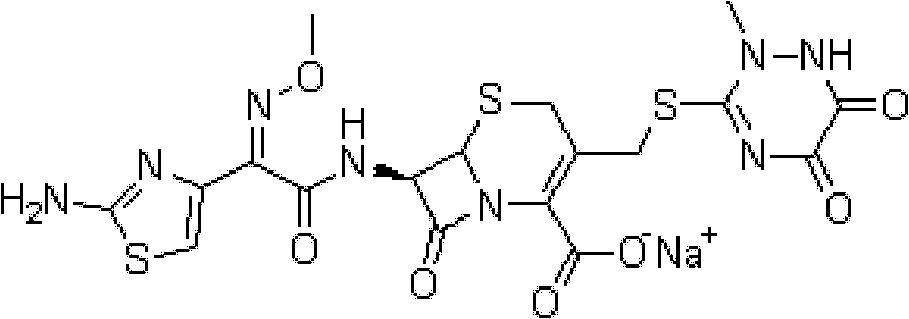
Method for refining ceftriaxone sodium crude product
A technology of ceftriaxone sodium and its refining method, which is applied in the field of chemical pharmacy, can solve problems such as instability of ceftriaxone sodium, high color grade of the product, and influence on the quality of ceftriaxone sodium, so as to improve safety and effectiveness, solvent Easy to get, cheap results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The refining method of embodiment 1, ceftriaxone sodium, the steps are as follows:
[0028] (1) Weigh 50 g of crude ceftriaxone sodium and 0.5 g of sodium metabisulfite, add a mixed solvent consisting of 10 ml of methanol and 60 ml of water, stir at room temperature for 30 min, add 1 g of activated carbon for decolorization for 20 min.
[0029] (2) The above solution is filtered through nitrogen pressure, and activated carbon and other impurities are removed by a microporous membrane. Then use the same proportion of water-methanol mixed solvent (5ml methanol and 30ml water) to wash the suction filter flask, wash the filter cake, and transfer to the crystallizer together.
[0030] (3) When the solution in the crystallizer reaches the crystallization temperature, start to add 50ml of fresh acetone dropwise. After the acetone is added dropwise, add sterile grade ceftriaxone sodium seed crystals. After growing the crystal for 30min, continue to add 370ml of acetone, and add...
Embodiment 2
[0033] The refining method of embodiment 2, ceftriaxone sodium, the steps are as follows:
[0034] (1) Weigh 50 g of crude ceftriaxone sodium and 1 g of sodium metabisulfite, add a mixed solvent consisting of 12 ml of methanol and 60 ml of water, stir at room temperature for 30 min, add 1 g of activated carbon for decolorization for 20 min.
[0035] (2) The above solution is filtered through nitrogen pressure, and activated carbon and other impurities are removed by a microporous membrane. Then use the same proportion of water-methanol mixed solvent (6ml methanol and 30ml water) to wash the suction filter flask, wash the filter cake, and transfer to the crystallizer together.
[0036] (3) When the solution in the crystallizer reaches the crystallization temperature, start to add 45ml of fresh acetone dropwise, add sterile grade ceftriaxone sodium seed crystals, continue to add 375ml of acetone after growing the crystal for 30min, add a total of 420ml of acetone, and crystalliz...
Embodiment 3
[0039] The refining method of embodiment 3, ceftriaxone sodium, the steps are as follows:
[0040] (1) Weigh 50 g of crude ceftriaxone sodium and 1 g of sodium metabisulfite, add a mixed solvent consisting of 14 ml of methanol and 60 ml of water, stir at room temperature for 30 min, add 1 g of activated carbon for decolorization for 20 min.
[0041] (2) The above solution was filtered by nitrogen pressure. Activated carbon and other impurities are removed by a microporous membrane. Then use the same proportion of water-methanol mixed solvent (7ml methanol and 30ml water) to wash the suction filter flask, wash the filter cake, and transfer to the crystallizer together.
[0042] (3) When the solution in the crystallizer reaches the crystallization temperature, start to drop 40ml of fresh acetone, add sterile grade ceftriaxone sodium seed crystals, continue to add 380ml of acetone after 30min of crystal growth, add a total of 420ml of acetone, and crystallize for 4.5 hours.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com