Preparation method of ceftriaxone sodium crystal and evaluation method of ceftriaxone sodium aqueous solution turbidity
A technology of ceftriaxone sodium and aqueous solution, which is applied in the field of preparation and evaluation of ceftriaxone sodium, can solve the problems of uncertain crystal form, uncontrollable quality and stability, and achieves mild conditions, good clarity, and occurrence of allergic reactions. low rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Get the raw material of ceftriaxone sodium (the condensation product sodium salt of 7-ACT and AE-active ester, without further purification) and prepare an aqueous solution, wherein the raw material of ceftriaxone sodium is 5 g and 40 ml of deionized water. Control the temperature of the aqueous solution at about 13°C, then add 4.1 g of sodium isooctanoate to the aqueous solution, stir the solution until it becomes clear, add 10 wt% dilute hydrochloric acid to adjust the pH value of the solution to 7.00, and continue stirring for 30 minutes to obtain the crude product of ceftriaxone sodium aqueous solution.
[0039] Get the above-mentioned crude product aqueous solution of ceftriaxone sodium, slowly add dehydrated ethanol therein under stirring, stop stirring just when turbidity occurs, and let stand for 25 minutes; then slowly add remaining dehydrated alcohol until a large amount of crystals are formed, The total amount is about 150ml.
[0040] The resulting crystals ...
Embodiment 2
[0042] The raw material of ceftriaxone sodium (the condensation product sodium salt of 7-ACT and AE-active ester, without further purification) was prepared into an aqueous solution, wherein the raw material of ceftriaxone sodium was 5 g and 15 ml of deionized water. Control the temperature of the aqueous solution at about 15°C, then add 10.0 g of sodium isooctanoate to the aqueous solution, stir the solution until it becomes clear, add 10 wt% dilute hydrochloric acid to adjust the pH value of the solution to 8.04, and continue stirring for 25 minutes to obtain the crude product of ceftriaxone sodium aqueous solution.
[0043]Get the above-mentioned ceftriaxone sodium crude product aqueous solution, slowly add acetone therein under stirring, stop stirring just when turbidity appears, and let it stand for 30 minutes; then slowly add remaining acetone until a large amount of crystals are formed, the total amount of acetone is about 180ml.
[0044] The resulting crystals are filt...
Embodiment 3
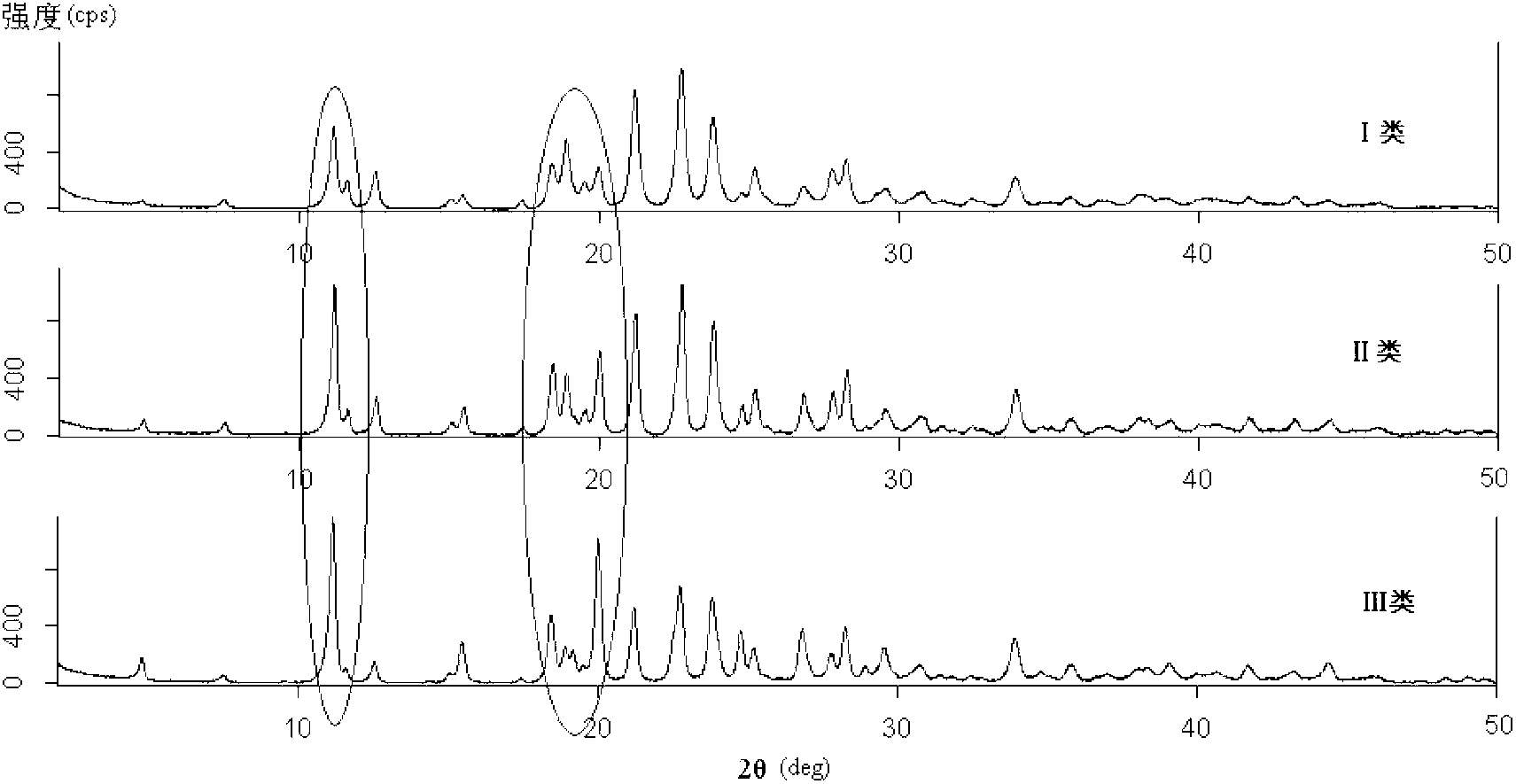
[0046] Using powder X-ray diffraction technology combined with cluster analysis method, more than 160 batches of ceftriaxone sodium samples for injection in the market were classified.
[0047] Powder X-ray diffraction experiment conditions are as follows:
[0048] Instrument: Rigaku Corporation D / max-2200 powder X-ray diffractometer.
[0049] Parameter setting: X-ray: CuK α1 Target; voltage 40KV; current 40mA. Slit width: 1deg, 1deg, 0.15mm. Scanning speed: 4deg / min. Scanning range (2θ): 2~50deg.
[0050] Analysis software: RINT 2000Series software. Integration conditions: peak width 0.05; minimum peak height 100.
[0051] Sample preparation: Take a sample of ceftriaxone sodium for injection, and press it into tablets by positive pressure on a glass plate.
[0052] The spectrograms of more than 160 batches of experimental samples obtained from powder X-ray diffraction experiments were analyzed with random RISM.Qualitative Analysis software, and the average diffraction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com