Method for removing cefaclor impurities
A cefaclor impurity, cefaclor technology, applied in the direction of organic chemistry, can solve the problems of slow dissolution, difficult to remove impurities, easy to cause allergic reactions, etc., to achieve the effect of reducing allergic reactions and reducing the incidence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
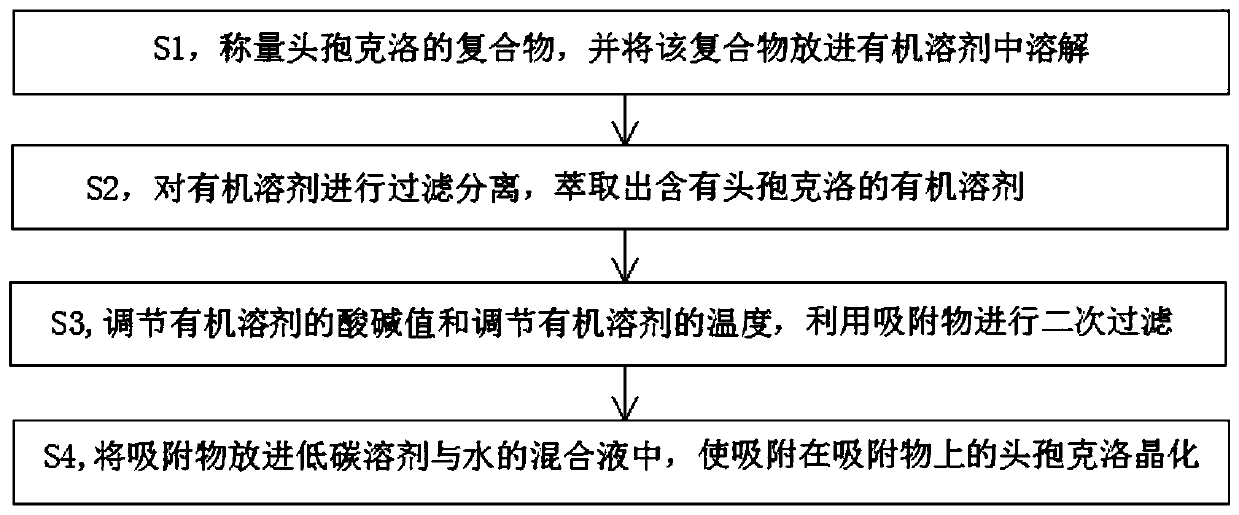
[0041] like figure 2 As shown, in this embodiment, the complex of 100g cefaclor is weighed, and the complex is put into 500ml of butyl fat solvent to dissolve and form a suspension, wherein the temperature of the butyl fat solvent is at 18-22°C, By stirring the suspension, the pH value of the suspension is adjusted to 0.8, so that the impurities are precipitated, and then by filtration, the impurities that are not dissolved in the butyl solvent are removed, and the filtered organic solvent is extracted to extract the cephalosporin containing Crowe's Organic Solvent.
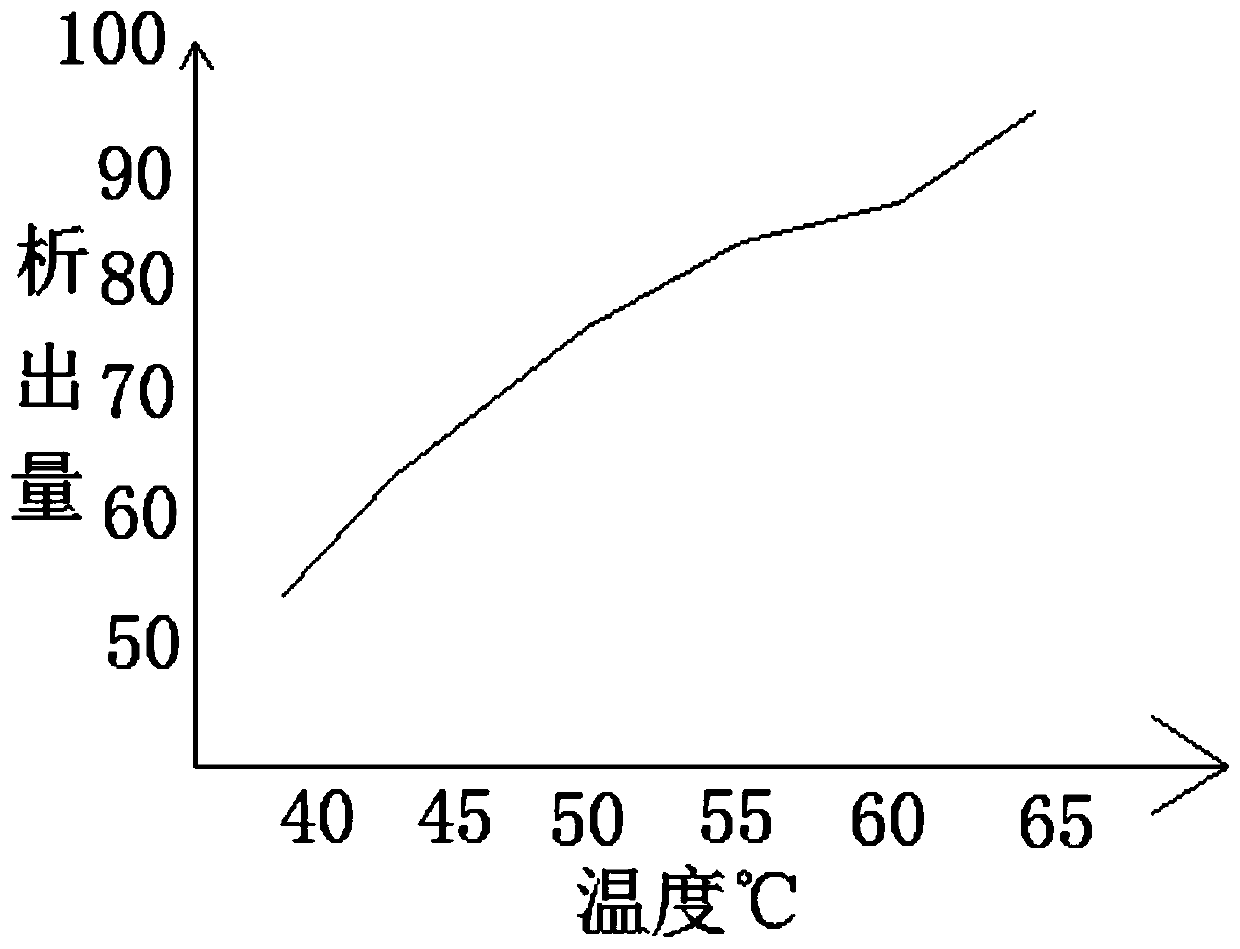
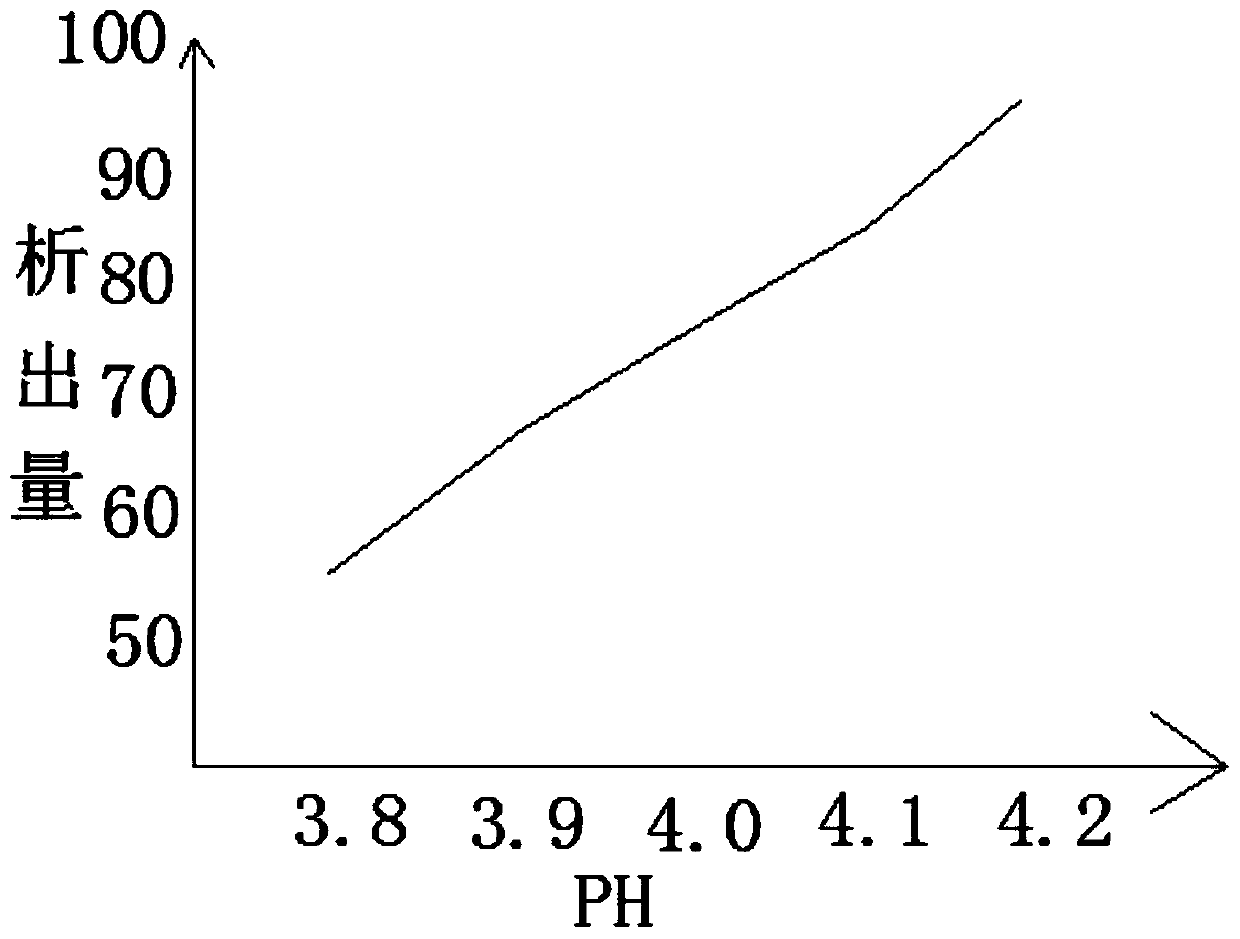
[0042] After putting in the activated carbon, adjust the pH value of the organic solvent to 3.8-4.2 by adding an alkaline reagent, wait for 1 hour for the substance to precipitate, and adjust the temperature of the organic solvent to 30°C, and perform secondary filtration. In the application, it can be filtered while stirring, so that the precipitate can be filtered better, and the filtered material can be put ...
Embodiment 2
[0050] like image 3 As shown, in the present embodiment, the complex of 100g cefaclor is weighed, and the complex is put into a butyl fat solvent to dissolve and form a suspension. By stirring the suspension, the pH value of the suspension is adjusted to 1.5, The impurities are separated out, and then the impurities insoluble in the butyl fat solvent are removed by filtration, and the filtered organic solvent is extracted to extract the organic solvent containing cefaclor.
[0051]After putting in the asbestos fiber, adjust the pH value of the organic solvent to 3.8-4.2 by adding an alkaline reagent, wait for 1 hour for the substance to precipitate, and adjust the temperature of the organic solvent to 35°C, perform secondary filtration, and In practical application, it is possible to filter while stirring, so that the precipitate can be filtered better, and the filtered material is put into a mixture of methanol, ethanol, acetone and water, wherein the low-carbon solvent and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com