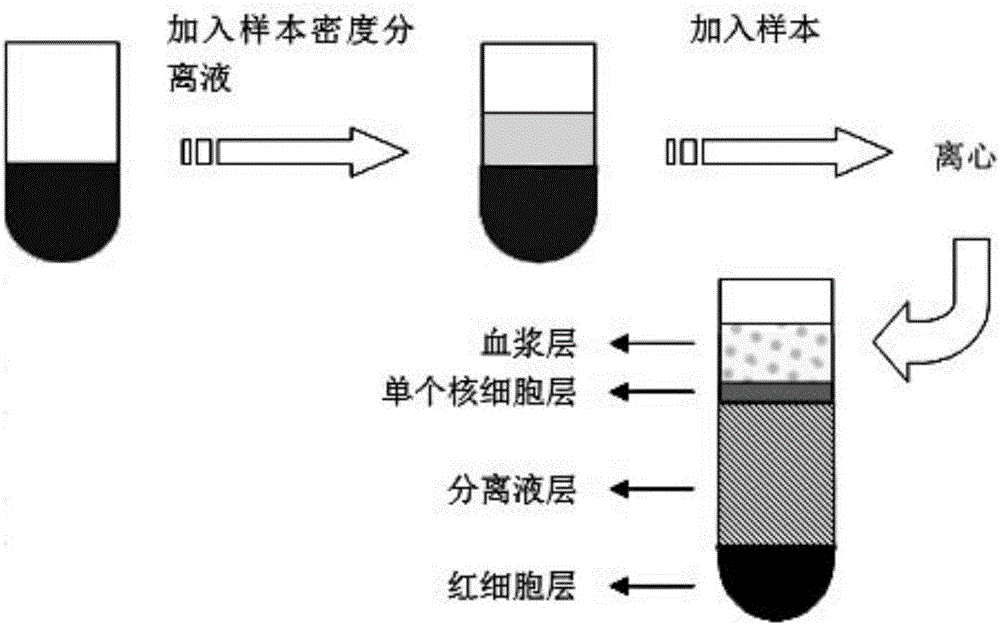
Patents
Literature
58results about How to "Reduce the incidence of allergic reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Three-dimensional kidney neoplasm surgery simulation method and platform based on computed tomography (CT) film
ActiveCN102982238AImprove surgical skillsImprove proficiencySpecial data processing applicationsSurgical riskGuideline
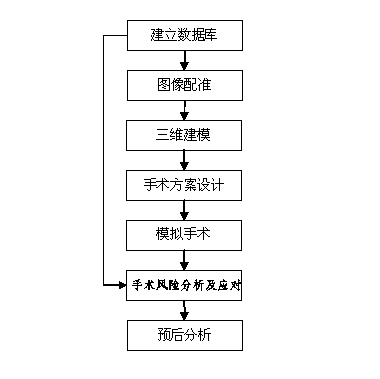
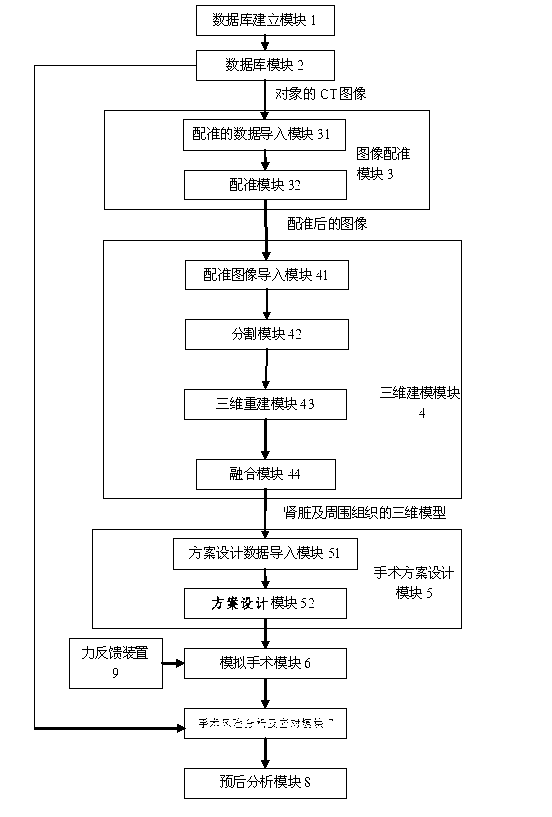
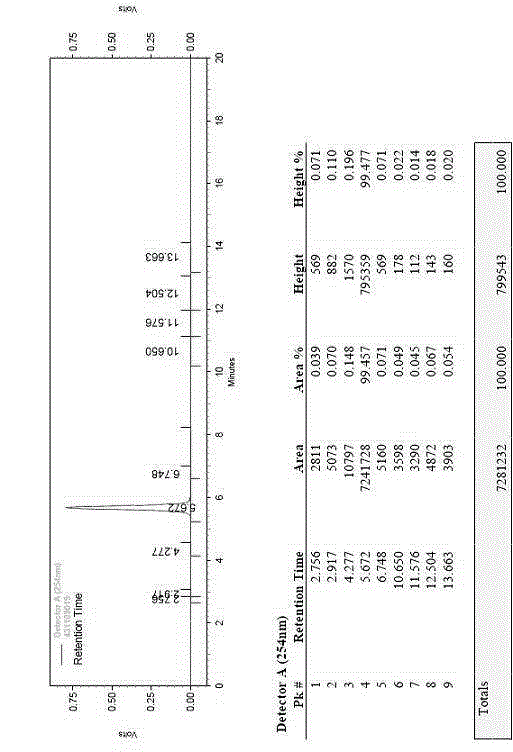
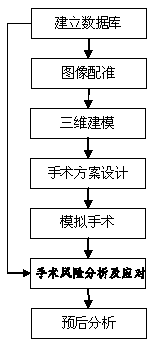
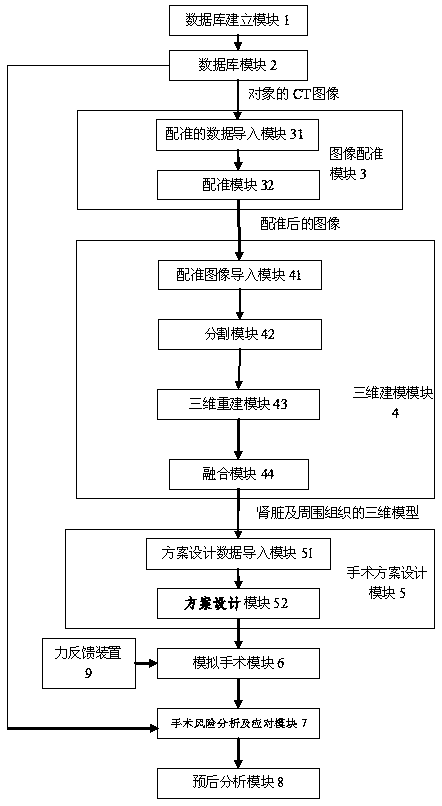
The invention discloses a three-dimensional kidney neoplasm surgery simulation method and a platform based on a computed tomography (CT) film. The method includes that firstly, a data base including object resources is built, multiple manners including CT film scanning are inputted into a CT faultage image, a sequence image is registered according to the sequence based on an outline gradient principle, a registered CT image is utilized to reconstruct segmentation tissue of an arterial phase, a venous phase and a lag phase by adopting of a three-dimensional reconstruction technology, and the segmentation tissue of the arterial phase, the venous phase and the lag phase are effectively fused in the same coordinate space. According to an imaging staging criteria and a treatment guideline of kidney neoplasm, and based on modeling results, various peration plan designing, operation stimulation, surgical risk analysis and response, and prognostic analysis are carried out, a personalized and benefit maximization treatment plan can be provided for an object, and skills and degree of proficiency of an operation can be improved, and the three-dimensional kidney neoplasm surgery simulation method can be used in medical teaching.
Owner:江苏瑞影医疗科技有限公司
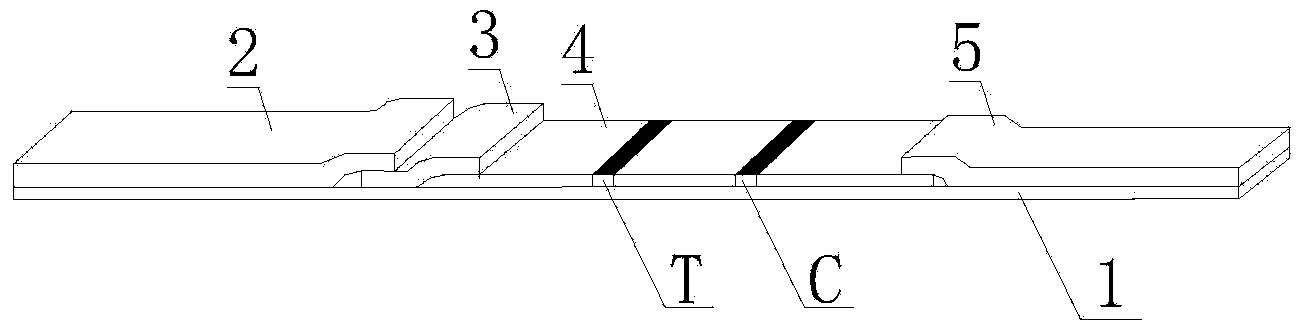
Streptococcus-A quantum dot immunochromatographic detection reagent card and preparation method thereof
ActiveCN103926403AImprove the detection rateOptimal treatment timeBiological material analysisFluorescence immunoassayFluorescence
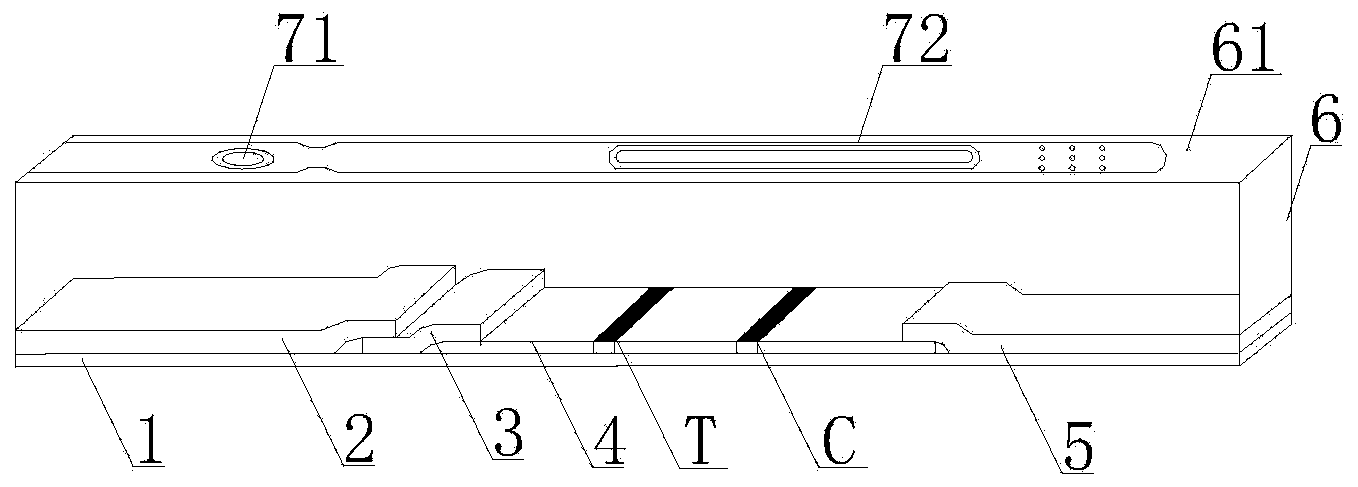
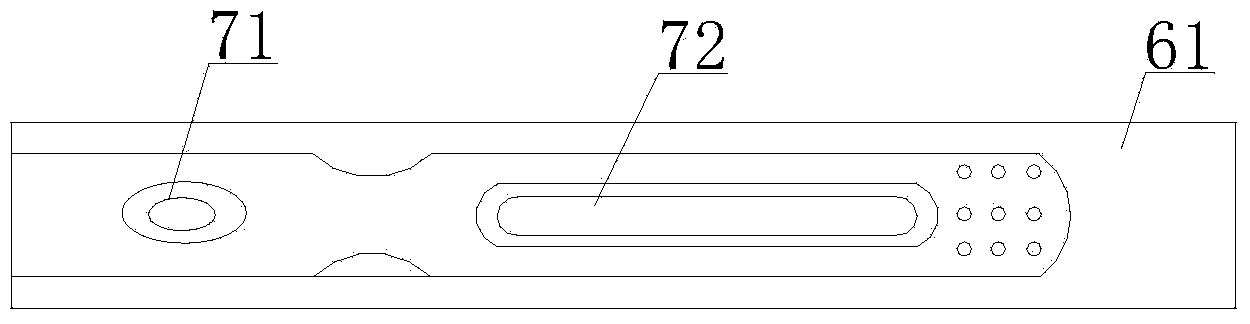
The invention discloses a streptococcus-A quantum dot immunochromatographic detection reagent card and a preparation method of the streptococcus-A quantum dot immunochromatographic detection reagent card, and aims to provide the streptococcus-A quantum dot immunochromatographic detection reagent card which is simple, high in sensitivity and reliable in detection results. The detection reagent card comprises a box body (6), wherein a piece of detection test paper is arranged in the box body (6); the detection test paper comprises a bottom plate (1); a sample pad (2), an anti-streptococcus-A quantum dot anti-body markup pad (3), a coating film (4) and a water absorption pad (5) are connected with the bottom plate (1) in sequence; a streptococcus-A quantum antibody detection area line T and a goat anti-rabbit polyclonal anti-body quality control area line C are arranged on the coating film (4); the two lines are arranged in parallel; the streptococcus-A quantum dot immunochromatographic detection reagent card and the preparation method thereof belong to the field of fluorescence immunoassay.
Owner:GUANGZHOU WEIMI BIOLOGICAL SCI & TECH
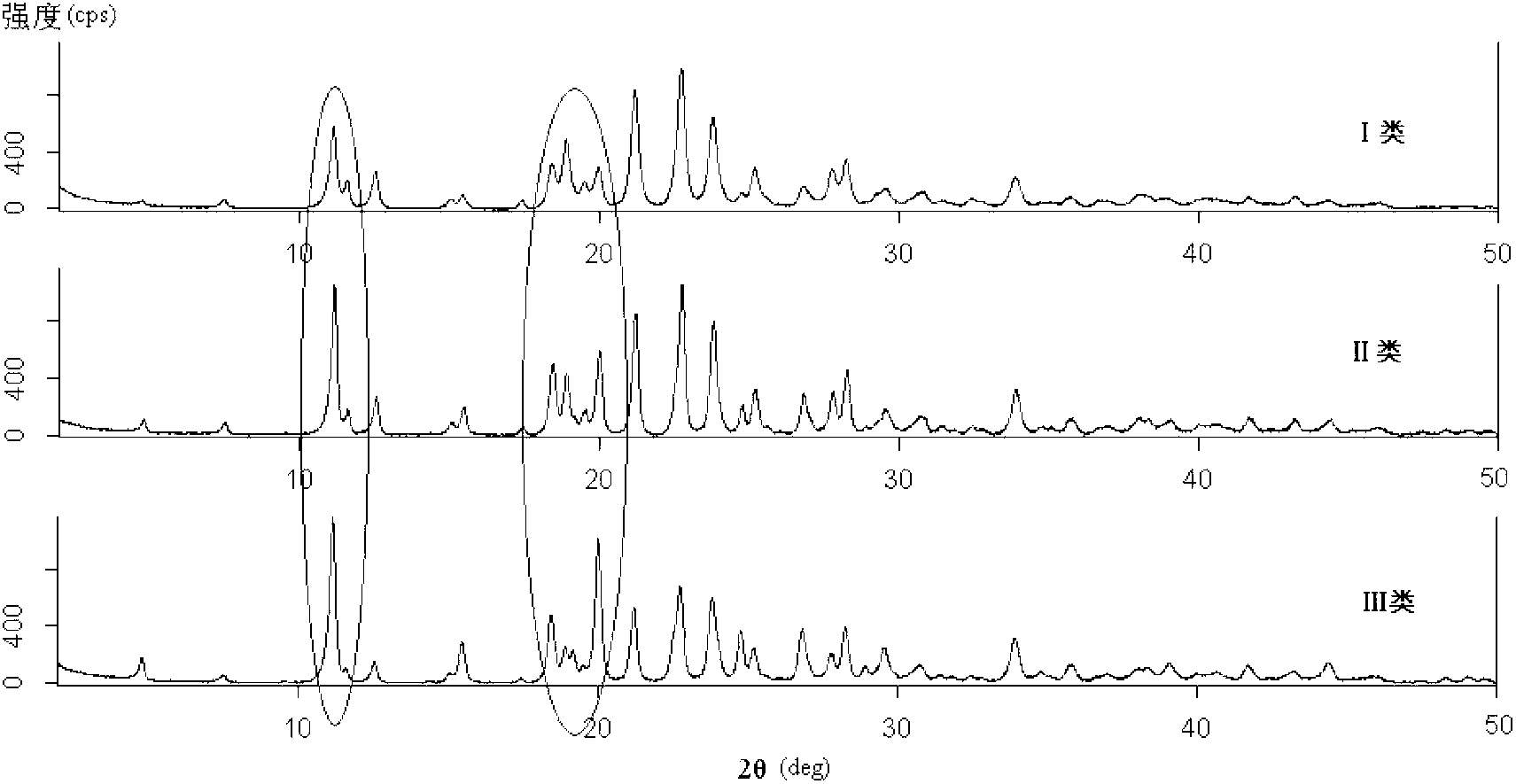
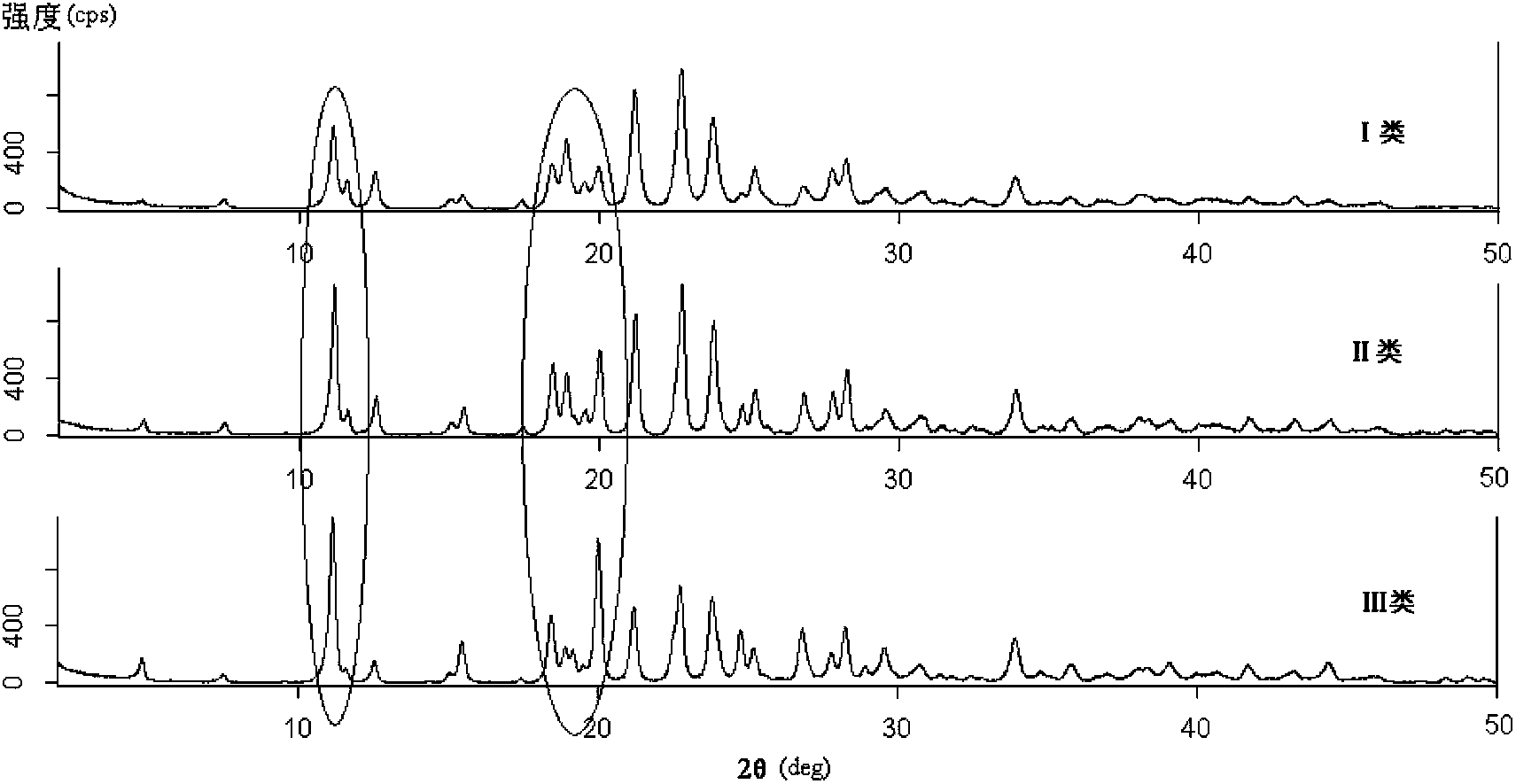
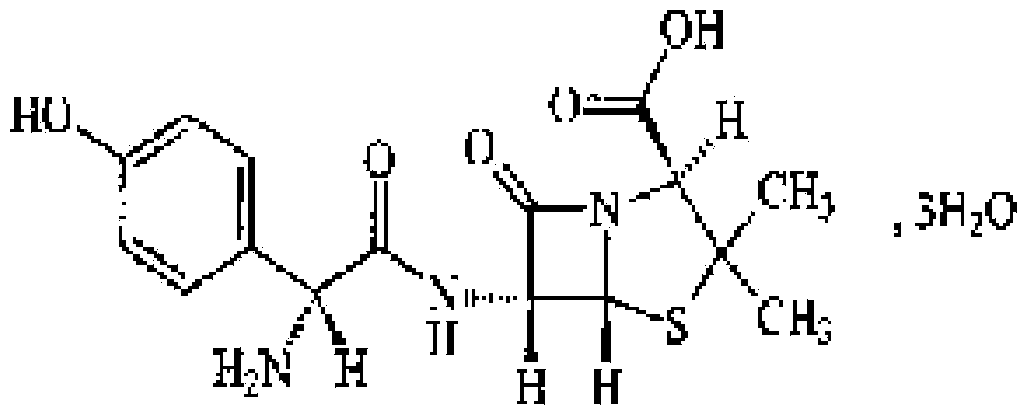
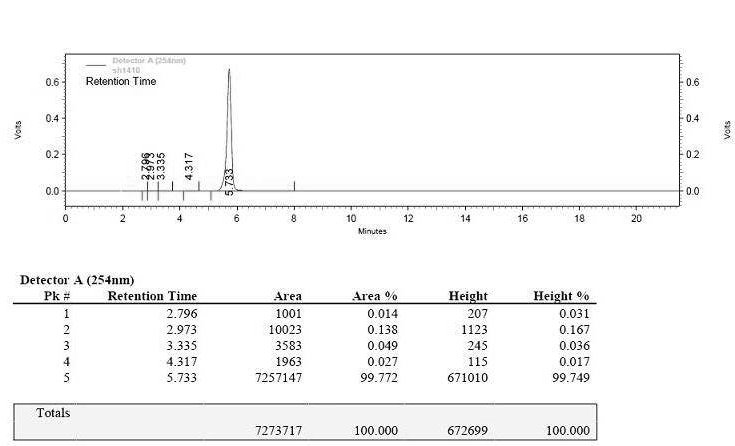
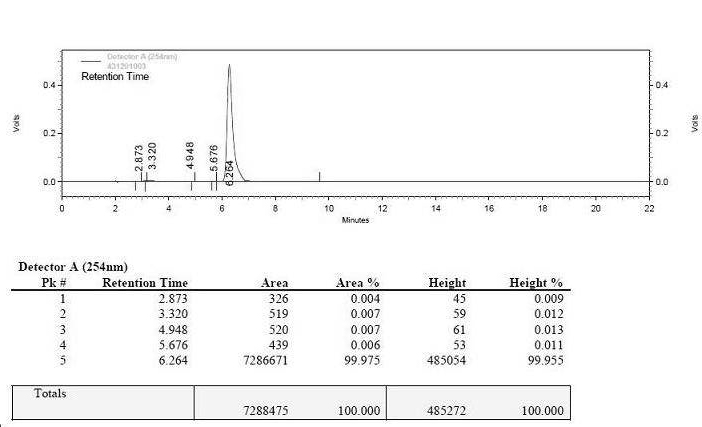
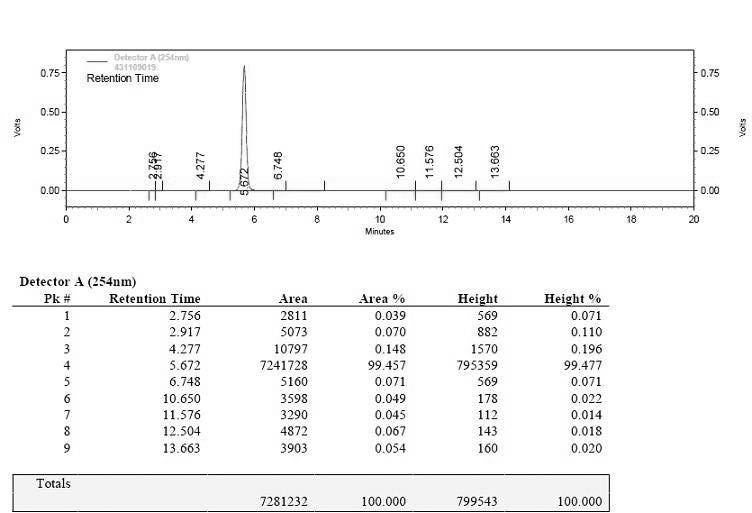
Preparation method of ceftriaxone sodium crystal and evaluation method of ceftriaxone sodium aqueous solution turbidity
ActiveCN102993215AReduce the incidence of allergic reactionsHigh clarityOrganic chemistryMaterial analysis using radiation diffractionCLARITYHypersensitive response
The invention relates to a preparation method of ceftriaxone sodium crystal, wherein the obtained ceftriaxone sodium is the crystal of the same subtype, and the subtype crystal has the best clarity, the lowest anaphylaxis occurrence rate and the highest safety in comparison with all crystals. The preparation method is simple in technology and excellent in reproducibility. The invention further relates to an evaluation method of ceftriaxone sodium aqueous solution turbidity, wherein a powder X-ray diffraction technology and a scanning electron microscope technology are used, in combination with statistical methods, a method for evaluating the ceftriaxone sodium crystal form is established, three subtypes of the ceftriaxone sodium crystal form is defined for the first time, and the relation between the subtypes and the aqueous solution turbidity is established, thus, rapid evaluation on crystal form and aqueous solution turbidity of ceftriaxone sodium samples becomes possible, and the method has great practical value.
Owner:YOUCARE PHARMA GROUP +1
Preparation method of ceftriaxone sodium crystal and evaluation method of ceftriaxone sodium aqueous solution turbidity
ActiveCN102993215BReduce the incidence of allergic reactionsHigh clarityOrganic chemistryMaterial analysis using radiation diffractionCLARITYX-ray
The invention relates to a preparation method of ceftriaxone sodium crystal, wherein the obtained ceftriaxone sodium is the crystal of the same subtype, and the subtype crystal has the best clarity, the lowest anaphylaxis occurrence rate and the highest safety in comparison with all crystals. The preparation method is simple in technology and excellent in reproducibility. The invention further relates to an evaluation method of ceftriaxone sodium aqueous solution turbidity, wherein a powder X-ray diffraction technology and a scanning electron microscope technology are used, in combination with statistical methods, a method for evaluating the ceftriaxone sodium crystal form is established, three subtypes of the ceftriaxone sodium crystal form is defined for the first time, and the relation between the subtypes and the aqueous solution turbidity is established, thus, rapid evaluation on crystal form and aqueous solution turbidity of ceftriaxone sodium samples becomes possible, and the method has great practical value.
Owner:YOUCARE PHARMA GROUP +1
Preparation method of thymopetidum injection
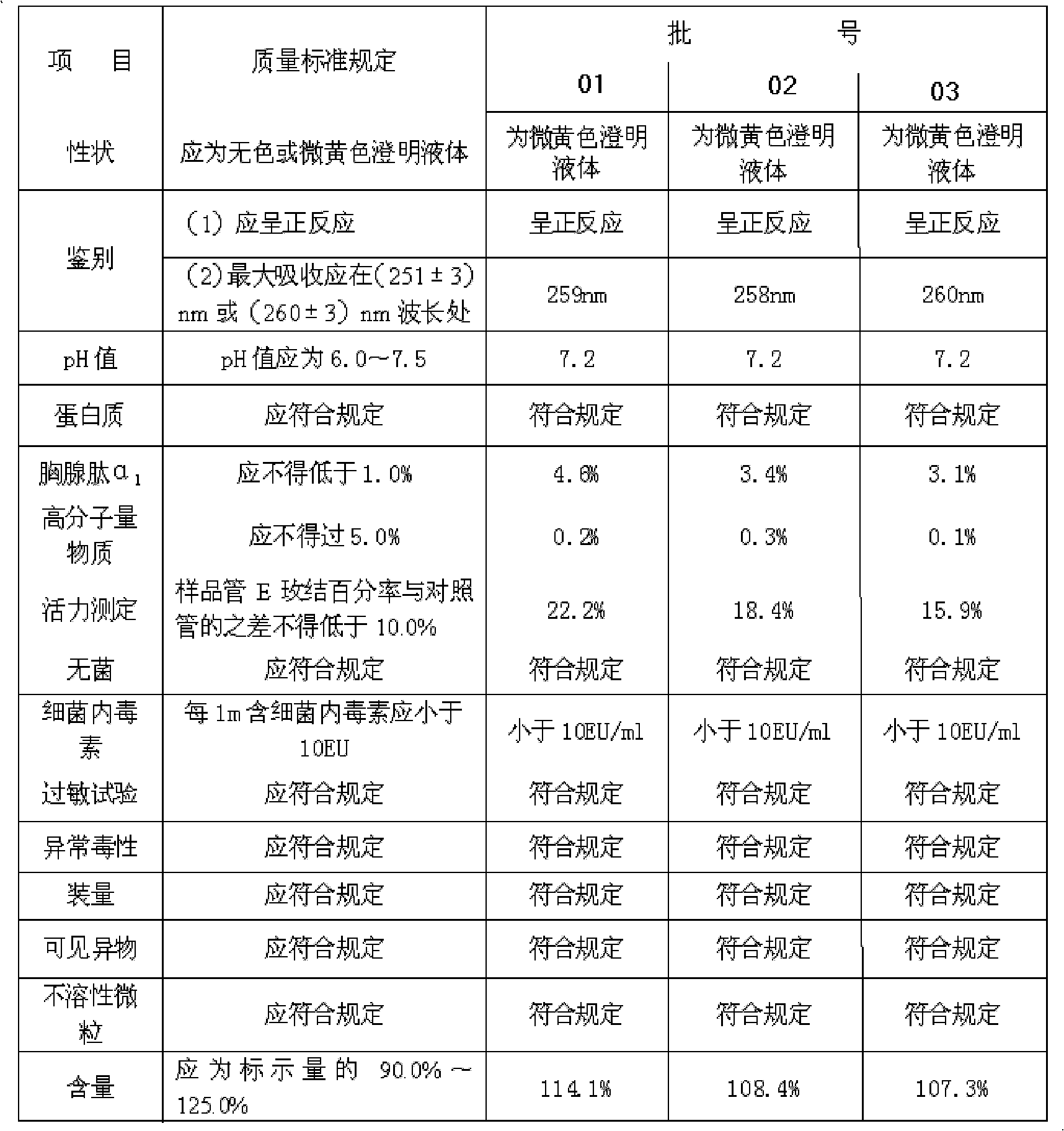
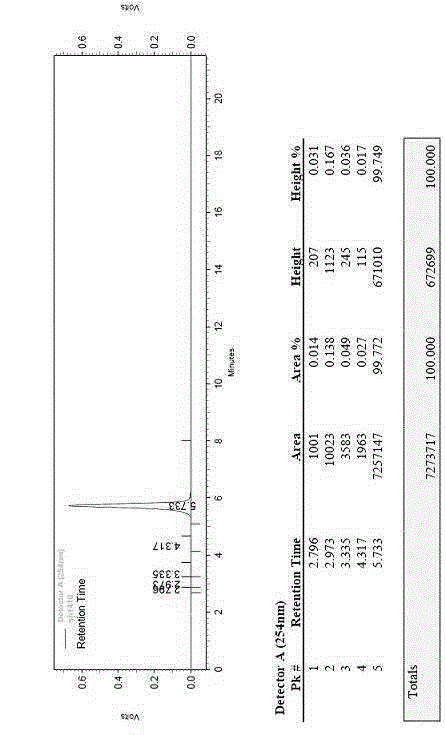
ActiveCN101934068AEfficient removalAffect clarityHormone peptidesPeptide/protein ingredientsUltrafiltrationSlurry
The invention discloses a preparation method of a thymopetidum injection, comprising the following steps of: mixing and grinding thymic tissues of calves or pigs and an aqueous sodium chloride solution; regulating a pH value to 3.5-4.5; freezing at the temperature from 15 DEG C below zero to 22 DEG C below zero; thawing and heating frozen mixed and ground fluid to the temperature of 33-37 DEG C; homogenizing the liquid to obtain homogenate; heating the homogenate to the temperature of 80 DEG C; insulating for 30 minutes; separating to obtain supernatant fluid; mixing the supernatant fluid and ethanol; standing; separating again; concentrating; regulating a pH value of concentrated fluid to 7.5-8.5; then, heating the concentrated fluid to the temperature of 80 DEG C; insulating for 30 minutes; cooling and centrifugally separating to obtain third supernatant fluid; after hyperfiltering and clarifying by an ultrafiltration membrane step by step, obtaining a thymopetidum solution; adding water for injection into the thymopetidum solution in order to regulate thymopetidum content of 2.5mg or 5mg or 10mg in each milliliter of the thymopetidum solution; adding a stabilizing agent; and obtaining the thymopetidum injection after being sterilized. In the prepared thymopetidum injection, the content of high polymer substances is smaller than 0.5 percent and is further lower than a standard rule of China.
Owner:武汉华龙生物制药有限公司
Ceftriaxone sodium compound and preparation method thereof
ActiveCN102617605BEasy to operateImprove product qualityAntibacterial agentsOrganic active ingredientsTriazineBULK ACTIVE INGREDIENT
The invention relates to a ceftriaxone sodium compound and a preparation method thereof. The compound comprises active ingredient ceftriaxone sodium, impurities and ceftriaxone polymer, wherein the content of ceftriaxone sodium is not less than 90.0% by calculation on the basis of an anhydride, the total weight percent of the impurities is not larger than 0.1% on the basis of the weight percent of ceftriaxone, the impurities comprise 7-ACT and triazine ring, and the weight percent of the ceftriaxone polymer is not larger than 0.1% on the basis of the weight percent of ceftriaxone. The preparation method of the compound is simple, economical and environment-friendly, the contents of the impurities and the polymer in a product are low, adverse reactions are remarkably reduced, and safety of clinical medication of a ceftriaxone sodium preparation can be guaranteed well.
Owner:石药集团中诺药业(石家庄)有限公司 +1
A three-dimensional renal tumor surgery simulation method based on CT film and its platform
ActiveCN102982238BIncrease authenticityAvoid wastingSpecial data processing applicationsThree dimensional simulationGuideline
The invention discloses a three-dimensional kidney neoplasm surgery simulation method and a platform based on a computed tomography (CT) film. The method includes that firstly, a data base including object resources is built, multiple manners including CT film scanning are inputted into a CT faultage image, a sequence image is registered according to the sequence based on an outline gradient principle, a registered CT image is utilized to reconstruct segmentation tissue of an arterial phase, a venous phase and a lag phase by adopting of a three-dimensional reconstruction technology, and the segmentation tissue of the arterial phase, the venous phase and the lag phase are effectively fused in the same coordinate space. According to an imaging staging criteria and a treatment guideline of kidney neoplasm, and based on modeling results, various peration plan designing, operation stimulation, surgical risk analysis and response, and prognostic analysis are carried out, a personalized and benefit maximization treatment plan can be provided for an object, and skills and degree of proficiency of an operation can be improved, and the three-dimensional kidney neoplasm surgery simulation method can be used in medical teaching.
Owner:江苏瑞影医疗科技有限公司
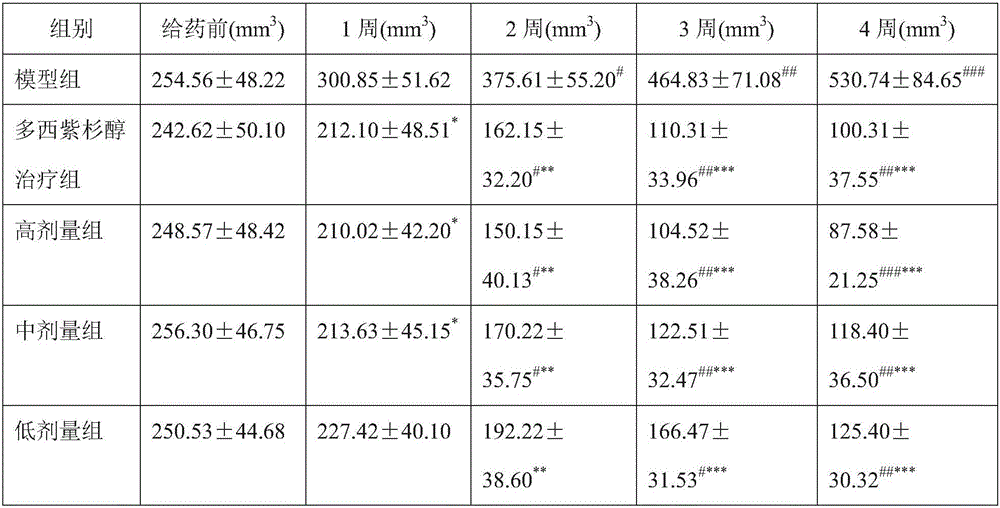
Polymeric micelle freeze-dried preparation of taxane anti-tumor drugs as well as preparation method and application of polymeric micelle freeze-dried preparation
ActiveCN106389355AGood molecular weight uniformityMeet the requirements for intravenous administrationPowder deliveryOrganic active ingredientsPolyesterFreeze-drying
The invention discloses a polymeric micelle freeze-dried preparation of taxane anti-tumor drugs as well as a preparation method and an application of the polymeric micelle freeze-dried preparation. The polymeric micelle freeze-dried preparation consists of a polyether / polyester segmented copolymer and taxane drugs, wherein the weight ratio of the polyether / polyester segmented copolymer to the taxane drugs is at (1-99) to 1; and in the polyether / polyester segmented copolymer, the molecular weight ratio of polyether to polyester is at 1 to (0.5-2). According to the taxane anti-tumor drug polymeric micelle freeze-dried preparation prepared by the invention, the physical stability of a redissolved solution is significantly increased; and meanwhile, the occurrence rate of a guinea pig allergic reaction in the taxane anti-tumor drug polymeric micelle freeze-dried preparation prepared from the polyether / polyester segmented copolymer which is subjected to ultrafiltration and freeze-drying treatment is obviously reduced, so that the safety of the taxane anti-tumor drug polymeric micelle freeze-dried preparation is further enhanced.
Owner:GUANGDONG ZHONGSHENG PHARMA
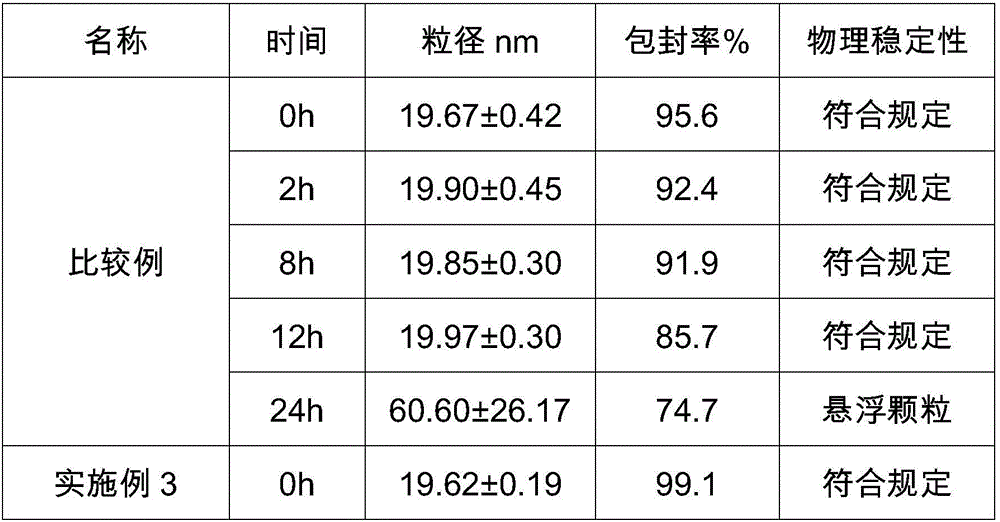
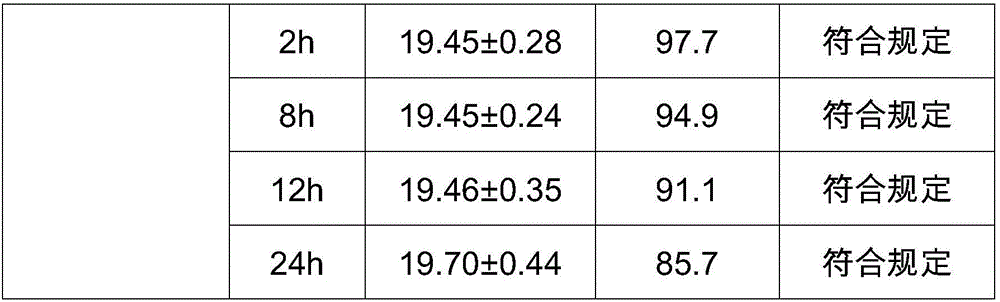
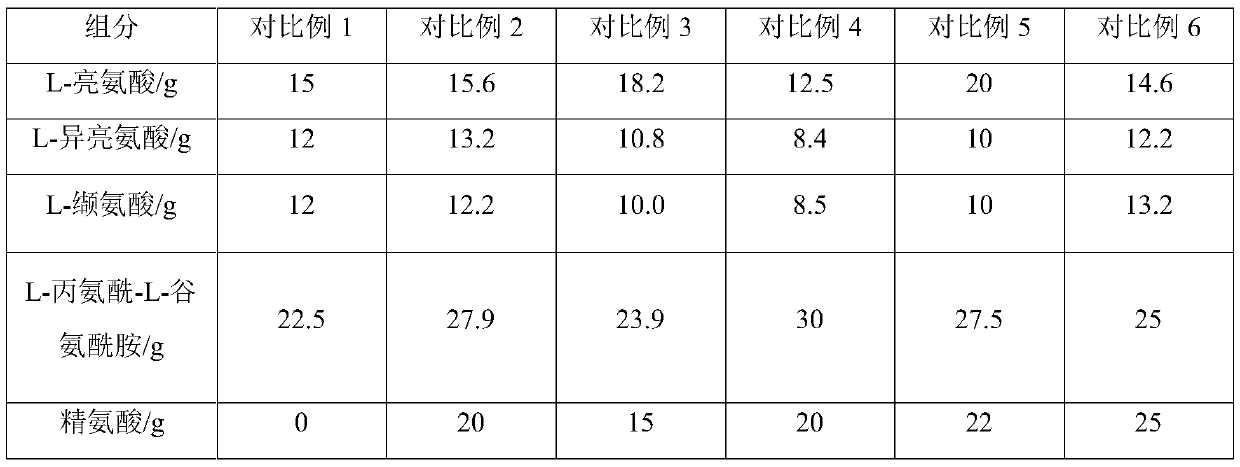
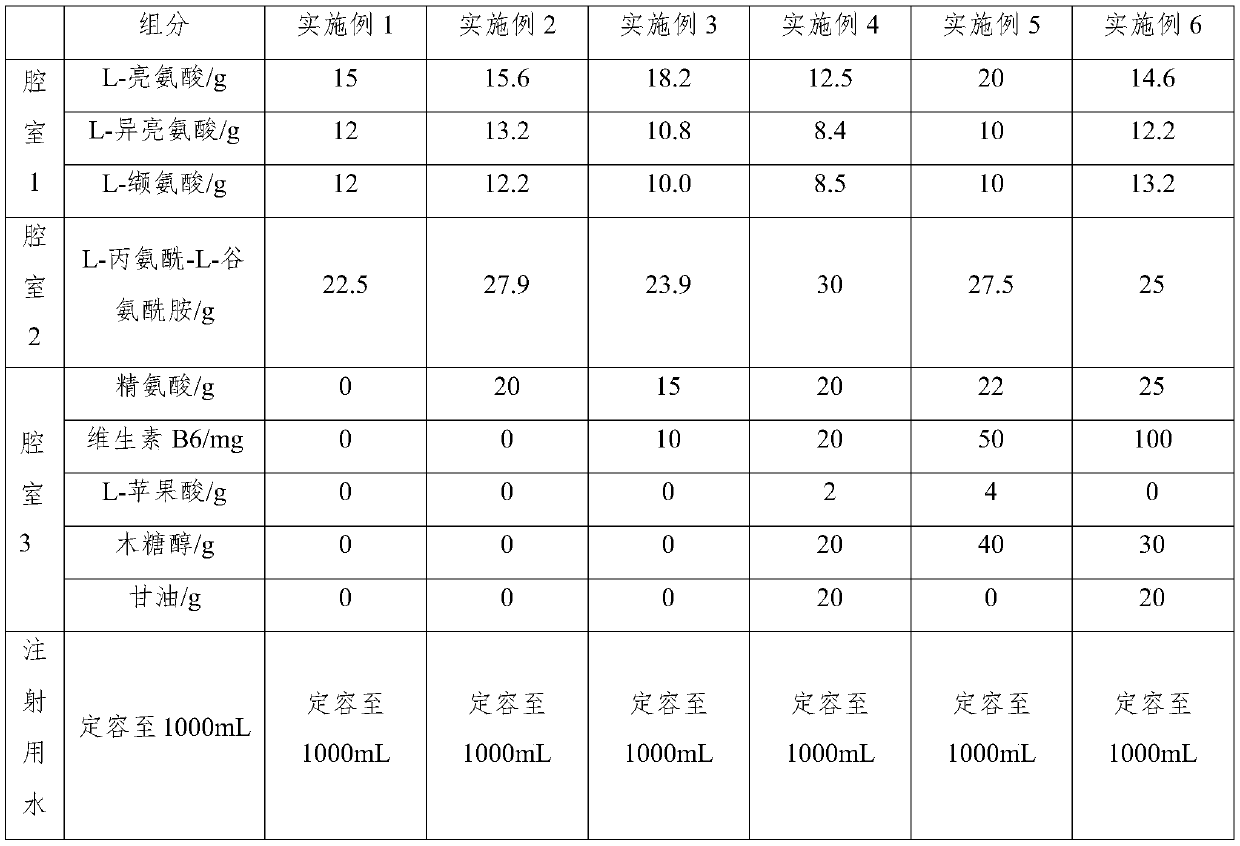
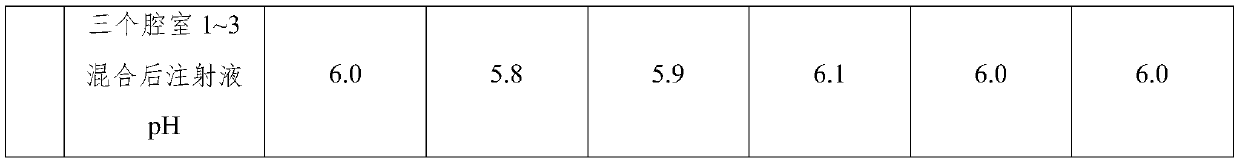
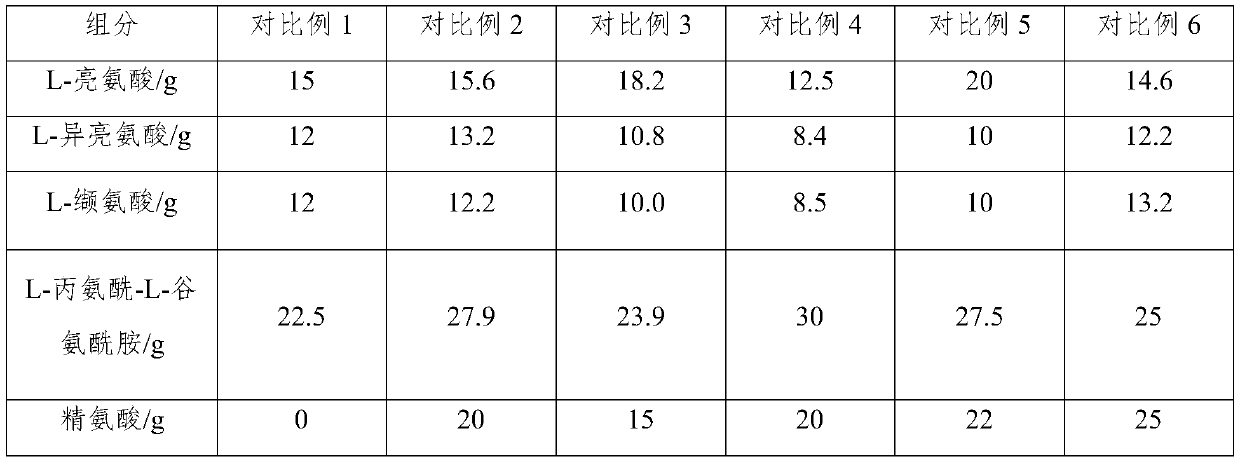
Compound amino acid dipeptide injection and preparation method and application thereof
InactiveCN110404048AAvoid decompositionImprove material and energy metabolismDipeptide ingredientsHydroxy compound active ingredientsDipeptideL-alanyl-l-glutamine
The invention belongs to the field of medicine, and provides a compound amino acid dipeptide injection, a preparation method thereof and an application thereof, the injection comprises three cavity bags, the three cavity bags are respectively separated by three bags and filled with a branched-chain amino acid solution, a L-alanyl-L-glutamine solution, and a mixed solution containing arginine hydrochloride and vitamin B6; wherein, in every 1000 mL of the injection solution, the total mass of the amino acid is 50 g to 130 g; and the branched chain amino acid mass accounts for 10 to 80% of the total mass of the amino acid; the mass of L-alanyl-L-glutamine is 5 to 70% of the total mass of the amino acid; the mass of arginine hydrochloride is 0 to 50% of the total mass of the amino acid; and the mass of the vitamin B6 is 0 to 1 g. The injection of the invention improves the stability and effectiveness of the product while providing product safety, and the technical scheme is simple and convenient for industrial production.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
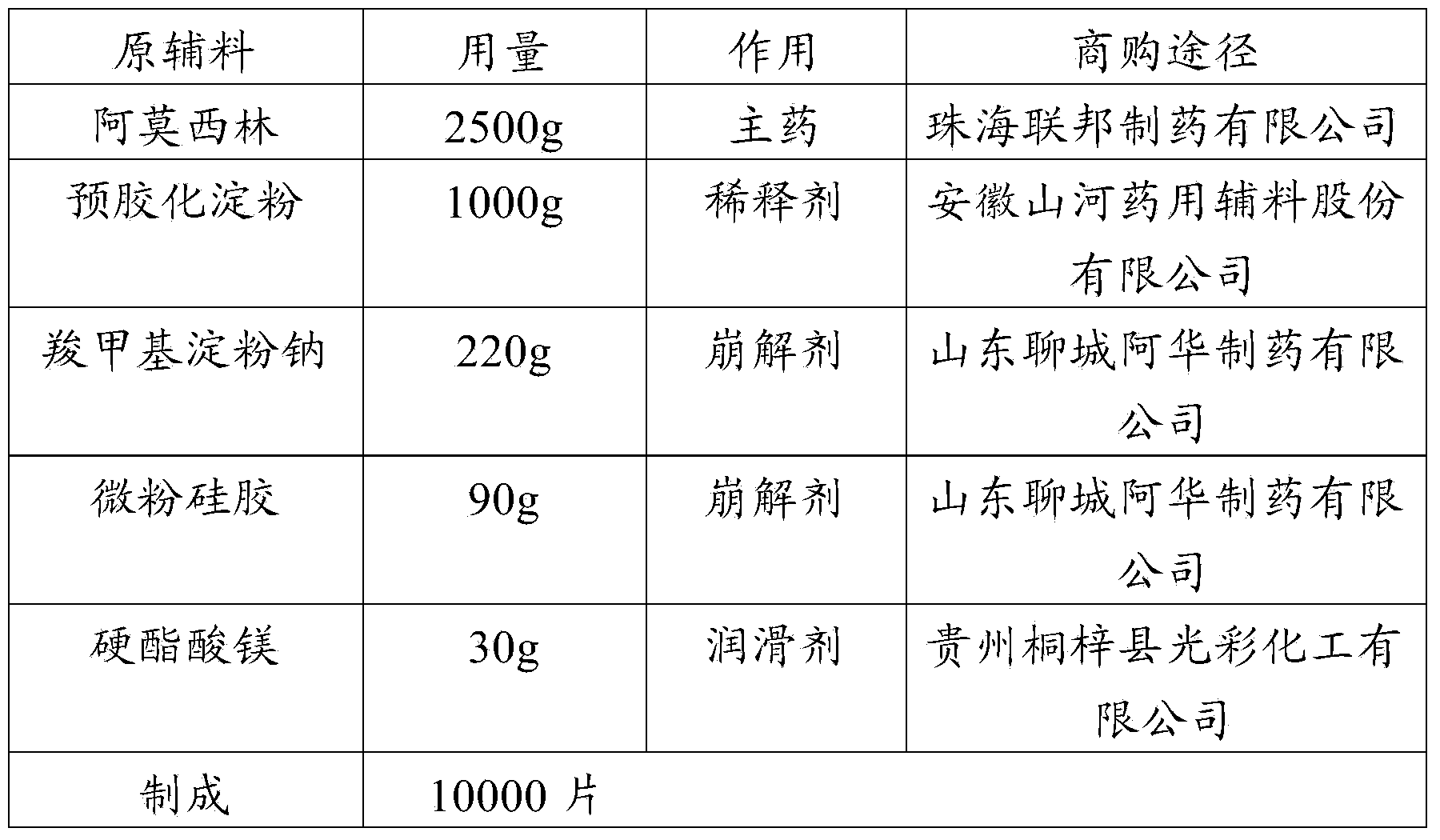
Stable amoxicillin tablet composition, as well as preparation method and application thereof
InactiveCN104173310ASolve solubilityReduce generationAntibacterial agentsPharmaceutical non-active ingredientsChemistryPatient compliance
The invention provides a stable amoxicillin tablet composition, as well as a preparation method and application thereof. The preparation process adopts a technology of directly tabletting powder. The amoxicillin tablet composition has the advantages that: the problems of amoxicillin tablet dissolution and product stability can be effectively solved, the influence of temperature and humidity on product quality can be avoided, generation of macromolecule impurities can be reduced, and the occurrence rate of anaphylactic reaction can be reduced, so that the clinical application safety can be improved, and the patient compliance can be improved.
Owner:HAIKOU PHARMA FACTORY
Anti-aging skincare product containing epidermal stem cell secretin and preparation method of anti-aging skincare product
ActiveCN105853341AImprove anti-aging effectReduce incidenceCosmetic preparationsToilet preparationsGlycerolEdgeworthia chrysantha
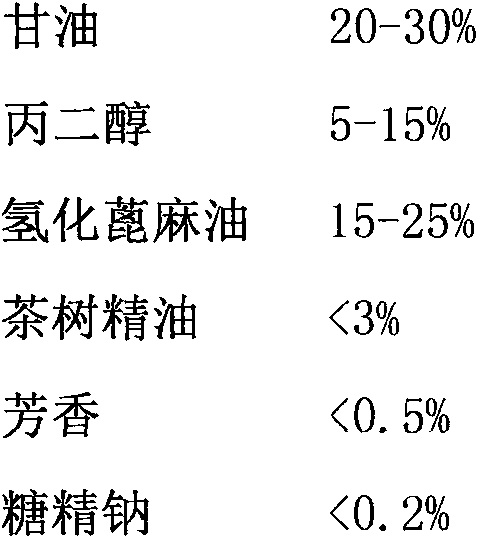
The invention belongs to the field of skincare products, in particular to an anti-aging skincare product containing epidermal stem cell secretin and a preparation method of the anti-aging skincare product. The skincare product is prepared from the following components by weight percentage: 0.000001-0.0005% of epidermal stem cell secretin, 2-8% of plant extract, 1-2% of squalane, 0.10-0.25% of EDTA-2Na, 5-10% of glycerol, 0.05-1% of poly-glutamic acid, 0.06-0.12% of K350, 2-6% of isopropyl myristate, 0.5-2% of cetearyl alcohol, 1-2% of polydimethylsiloxane and the balance of water, wherein the plant extract is prepared from semen adenantherae pavoninae, edgeworthia chrysantha, radix acanthopanacis senticosi, ilicis rotundae cortex and fructus rubi. The skincare product provided by the invention can significantly improve skin aging, delay aging, reduce the incidence of adverse reactions, is simple in preparation method and stable in property.
Owner:广东省科玮智丽生物医药有限公司
Material for preventing diaper dermatitis and production method thereof
ActiveCN102512705BRelieve symptomsReduce the number of spawnsAbsorbent padsBandagesParticle compositionDiaper Dermatitis
The invention relates to a material for preventing diaper dermatitis and a production method thereof. The material for preventing diaper dermatitis is a composite rolled material with a sandwich structure; a granular composition for preventing and treating diaper dermatitis is clamped in a composite layer; and the composite rolled material is formed by performing hot-press composition by a hot roller and slitting. The invention has the characteristics that the material can prevent diaper dermatitis, and is not allergic, stable, continuous and effective and is convenient to use; and the material is applied to baby diapers.
Owner:中健生命科技(深圳)有限公司
Folium artemisiae argyi flavone freckle-removing gel
InactiveCN107744483ASimple production processReduce consumptionCosmetic preparationsToilet preparationsOrganic solventAnaphylactic reactions
The invention belongs to the field of cosmetics with special purposes and in particular relates to folium artemisiae argyi flavone freckle-removing gel. The folium artemisiae argyi flavone freckle-removing gel is characterized by being prepared from the following components in parts by weight: 23 percent to 26 percent of folium artemisiae argyi extract, 4.5 percent to 5.5 percent of water-solubleazone, 0.8 percent to 1.2 percent of a gelling agent, 1 percent to 1.5 percent of a humectant, 2 percent to 3 percent of a surfactant, 0.5 percent to 1 percent of a co-surfactant and the balance of purified water which is added until the content is 100 percent. The folium artemisiae argyi flavone freckle-removing gel provided by the invention has the advantages that a production technology of thefolium artemisiae argyi extract is simple, the consumption of organic solvents is less and the variety of flavones in a product is abundant; the content of the flavone in the folium artemisiae argyi extract is detected by adopting an ultraviolet spectrophotometry and is 21.3 percent to 27.5 percent. Folium artemisiae argyi oil has an anti-allergic effect; when the content of the folium artemisiaeargyi oil is relatively low, the anaphylactic reaction probability in a utilization process is easy to reduce; after a transdermal agent azone is added, the folium artemisiae argyi flavone can easilydirectly act on freckles through skin and the metabolism of skin cells is accelerated, so that the effects of realizing freckle fading and preventing new freckles from being generated are realized.
Owner:马述腾
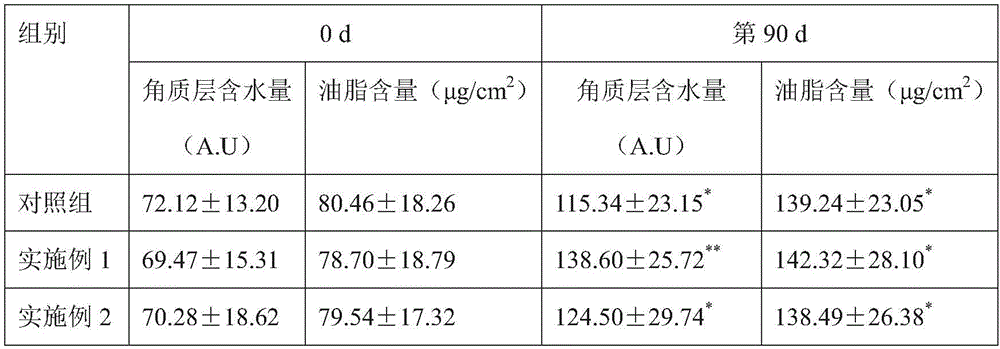
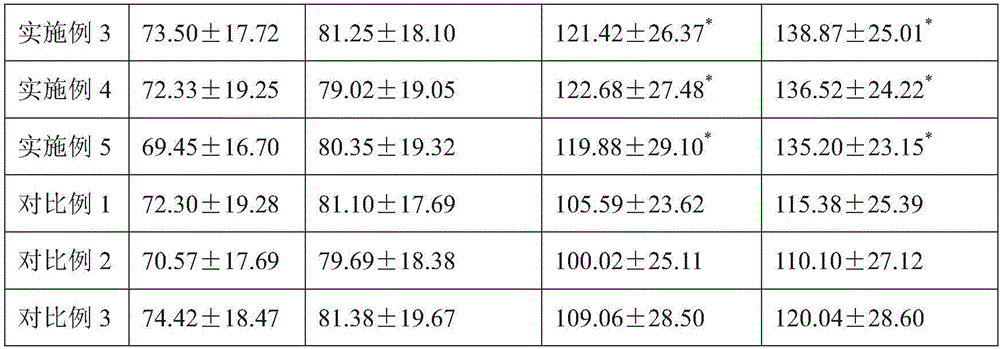
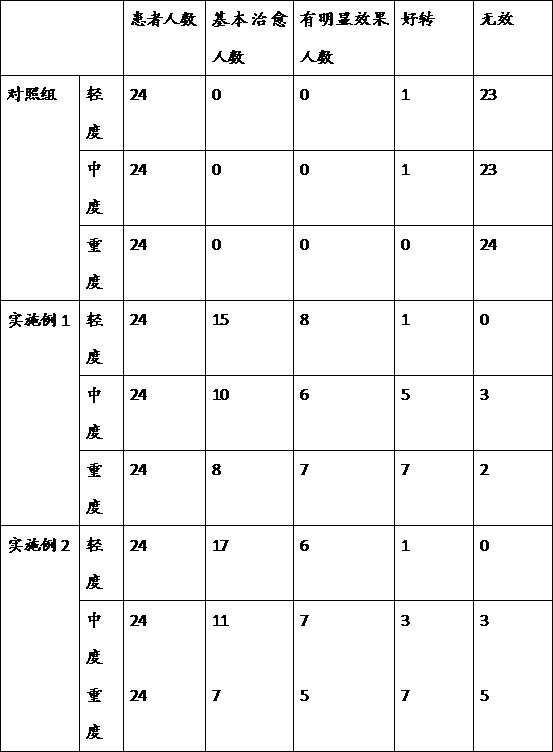
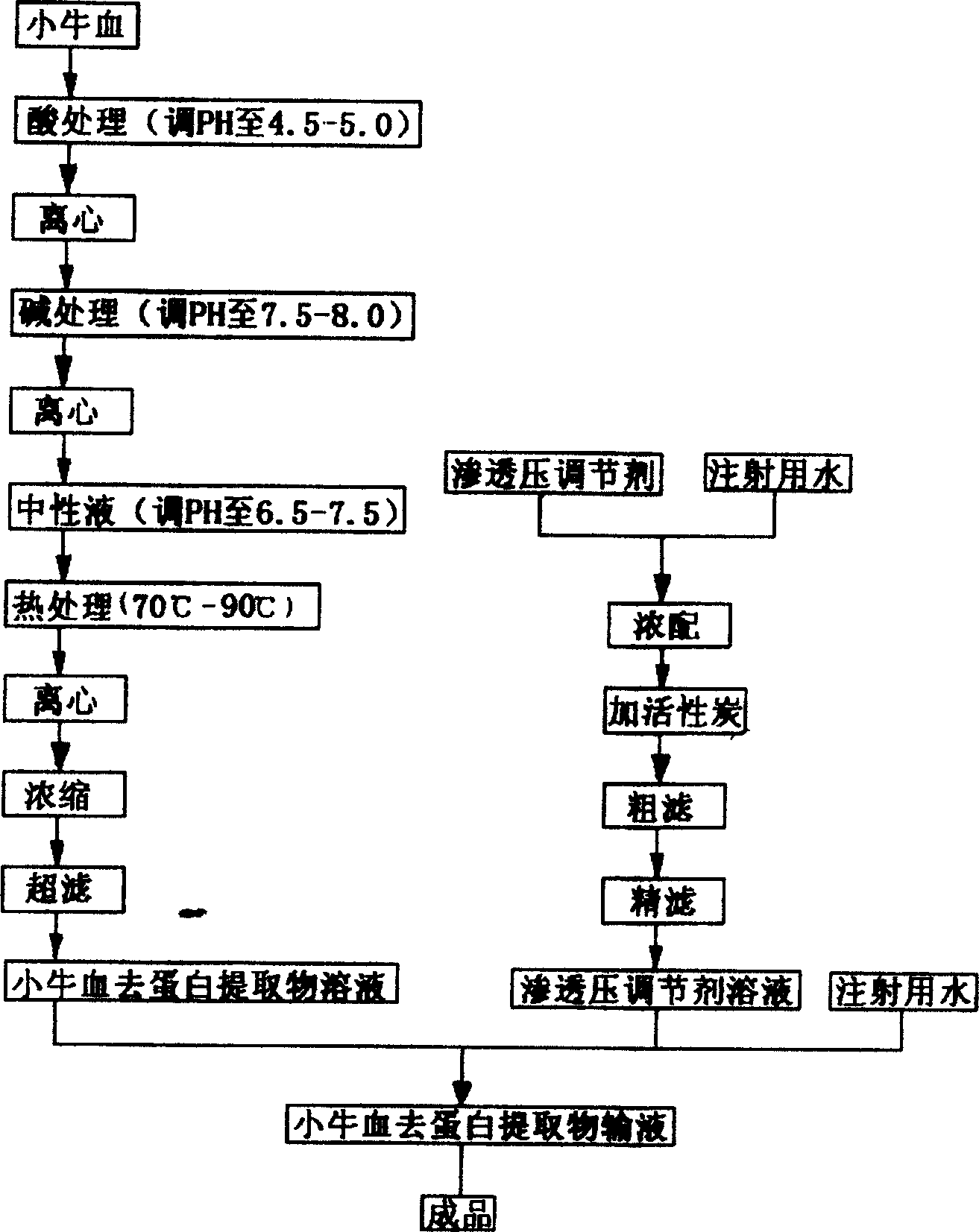
Veal-blood protein-removed extract infusion and its preparation process
ActiveCN1251689COvercome the defects that are prone to clinical allergic reactionsReduce precipitationNervous disorderPeptide/protein ingredientsAdditive ingredientDrug biological activity
The invention is calf dehemoglobinize extract transfusion and its manufacturing method. The amino acid and small peptide in the extract of the calf dehemoglobinize can be polymerized into high molecular peptide and large molecular weight peptide, thus the medicine has antigenicity and reduces the biology activity of the medicine, and causes the increase of clinic hypersusceptibility. The ingredients of the calf dehemoglobinize are: the calf dehemoglobinize extract and osmotic pressure regulator, the calf dehemoglobinize extract is extracted from the calf blood or blood serum, the extract solid contains nitrogen and amino acid, its weight shares are: amino acid is 20.0-30.0, the nitrogen 12.5-18.75, the osmotic pressure regulator 50-100, pH value is 5.5-8.0, the product is a little yellow or yellow transparent liquid. The product can be applied to cure cerebral ischemia, cerebral dementia, brain trauma and incompetence of brain fucntiosn, and so on, they are all brain cell metabolism handicap diseases; it also can be applied to cure the twig artery, vein circulation obstruction, and artery angiopathy.
Owner:江卫世
Method for improving yield of hyaluronic acid with high molecular weight
The invention relates to a method for improving yield of hyaluronic acid with a high molecular weight. The method comprises the following steps: (1) performing primary treatment on fermentation liquor; (2) purifying the fermentation liquor; and (3) drying hyaluronic acid. The purification method provided by the invention is excellent in purification effect, particularly high in protein removal rate, the yield of hyaluronic acid products is high and can reach 8-10g / L, the recovery rate can be 95%, the service performance is excellent, the solubility is high, and the light transmittance reaches 99%.
Owner:TIANJIN KANGTING BIOLOGICAL ENG GRP CO LTD
Traditional Chinese medicine composition
The invention discloses a traditional Chinese medicine composition. The composition is prepared from the following raw materials in parts by weight of 20-60 parts of fibrilia, 15-35 parts of vegetableoil, 20-28 parts of animal oil, 5-15 parts of herba lycopodii, 10-30 parts of radix angelicae pubescentis and 10-35 parts of radix salviae miltiorrhizae. The composition has the following beneficialeffects that the vegetable oil and the animal oil are mixed to improve the permeability and adhesion of the medicines to the skin, especially the adhesive animal oil is sticky and changes patient allergic components in a formula after being mixed with the medicines, the content of the allergic components is reduced to current 0.5% from original 1.5%, and the composition has the strange effect forkneesynovitis, spur and meniscus tear.
Owner:乌兰托娅 +1
Lip balm
InactiveCN103767989ASimple ingredientsLess additivesCosmetic preparationsToilet preparationsVegetable oilAllergic response
The invention discloses a lip balm. The lip balm comprises the following raw materials in parts by weight: 20-30 parts of honey, 6-10 parts of vegetable oil, 2-8 parts of Vaseline, 1-2 parts of vitamin E, and 1-3 parts of emulsifier, preferably 25 parts of honey, 8 parts of vegetable oil, 5 parts of Vaseline, 2 parts of vitamin E, and 1.5 parts of emulsifier, wherein the vegetable oil is one or more of coconut oil, sunflower seed oil and calendula oil; the emulsifier is dodecyl trimethyl ammonium chloride. The lip balm provided by the invention is simple in components, few in additives, good in nourishing effect and safe and non-toxic, can be used for a long time, and can greatly reduce the occurrence rate of allergic responses.
Owner:SUZHOU CITY BANGCHENG ELECTRICITY TECH
Application of ulinastatin in preparation of drugs for treating prostatic cancer
InactiveCN105816862ASmall toxicityImprove securityPowder deliveryPeptide/protein ingredientsProstate cancerCurative effect
The invention belongs to the technical field of medicine, and specifically discloses an application of ulinastatin in the preparation of drugs for treating prostatic cancer. The test results show that ulinastatin can prominently prevent the weight loss of naked mice with prostatic cancer and inhibit the increasing of tumor volume; and moreover, ulinastatin can increase the expression of TNF-alpha of naked mice with prostatic cancer to promote apoptosis of tumor cells and reduce the expression of NF-kB to reduce the generation of related inflammation factors, and thus finally prevents prostatic cancer. Ulinastatin has the advantages of less adverse reactions, low side and toxic effect, high safety, low cost, and good curative effect on prostatic cancer.
Owner:GUANGDONG TECHPOOL BIO-PHARMA CO LTD
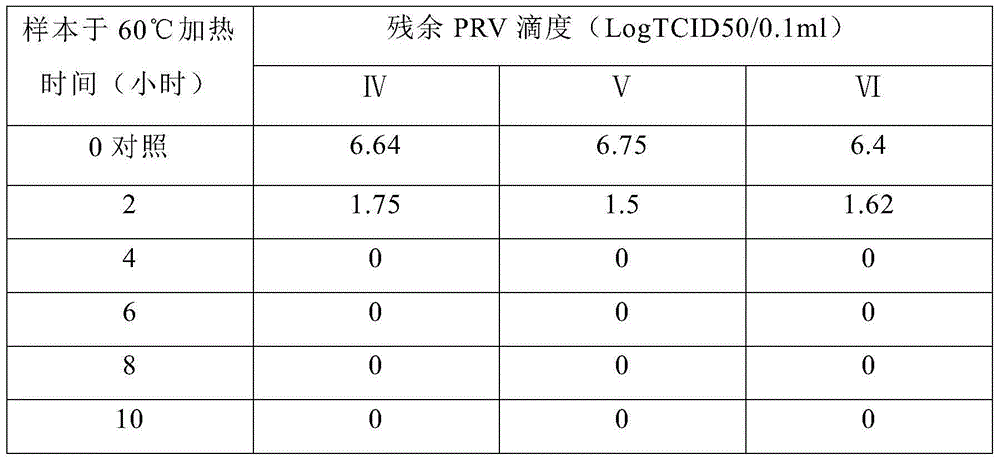
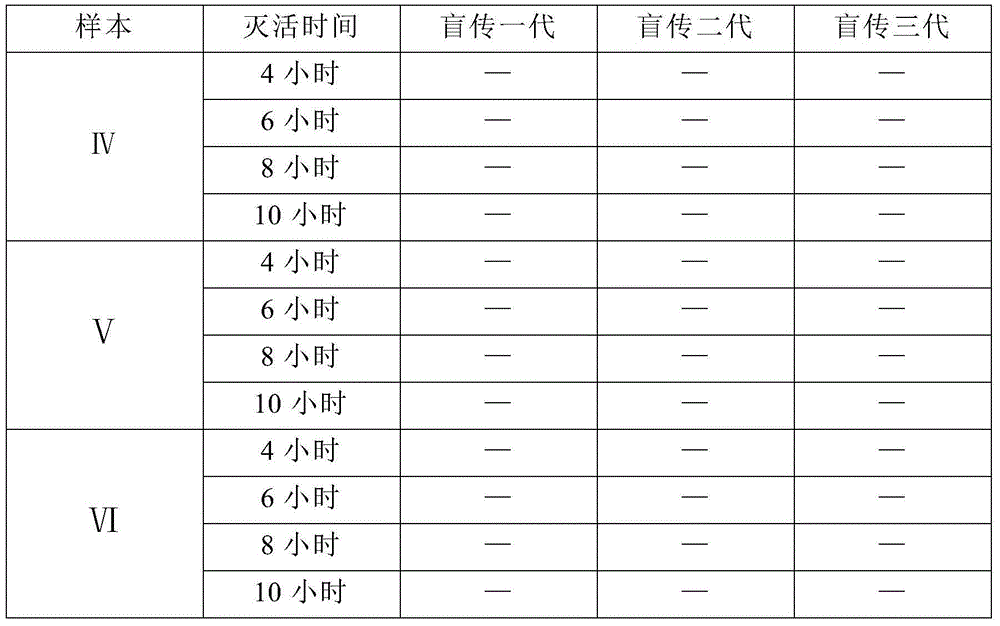
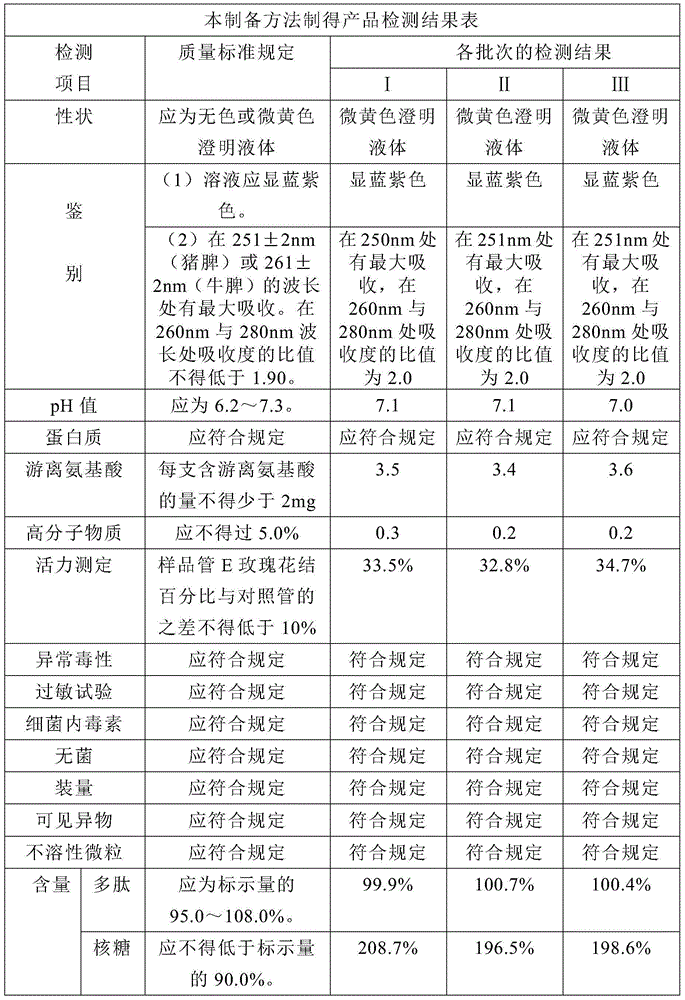
Preparation method of transfer factor and preparation method of injection thereof
ActiveCN104644673AEnsure safetyReduce allergensPeptide/protein ingredientsPharmaceutical delivery mechanismUltrafiltrationChemistry
The invention discloses a preparation method of a transfer factor. The preparation method of the transfer factor is characterized by comprising the steps: pre-processing, homogenation, repeated freeze thawing, centrifugation, ethanol precipitation, primary ultra-filtration, virus inactivation and secondary ultra-filtration. The invention also discloses a preparation method of a transfer factor injection. According to an ethanol precipitation method and a virus deactivation method which are not adopted in a traditional process, various impurity proteins are removed, and various animal source viruses are effectively removed, so that the security of products can be effectively guaranteed, sensitive sources are reduced, and the security is improved; meanwhile, by adopting an ethanol precipitation method, the impurity proteins are removed, so that the ultra-filtration load of a subsequent ultrafiltration membrane can be reduced, and the service life of the ultrafiltration membrane is prolonged.
Owner:武汉华龙生物制药有限公司
Ceftriaxone sodium compound and preparation method thereof
ActiveCN102617605AEasy to operateImprove product qualityAntibacterial agentsOrganic active ingredientsTriazineBULK ACTIVE INGREDIENT
The invention relates to a ceftriaxone sodium compound and a preparation method thereof. The compound comprises active ingredient ceftriaxone sodium, impurities and ceftriaxone polymer, wherein the content of ceftriaxone sodium is not less than 90.0% by calculation on the basis of an anhydride, the total weight percent of the impurities is not larger than 0.1% on the basis of the weight percent of ceftriaxone, the impurities comprise 7-ACT and triazine ring, and the weight percent of the ceftriaxone polymer is not larger than 0.1% on the basis of the weight percent of ceftriaxone. The preparation method of the compound is simple, economical and environment-friendly, the contents of the impurities and the polymer in a product are low, adverse reactions are remarkably reduced, and safety of clinical medication of a ceftriaxone sodium preparation can be guaranteed well.
Owner:石药集团中诺药业(石家庄)有限公司 +1
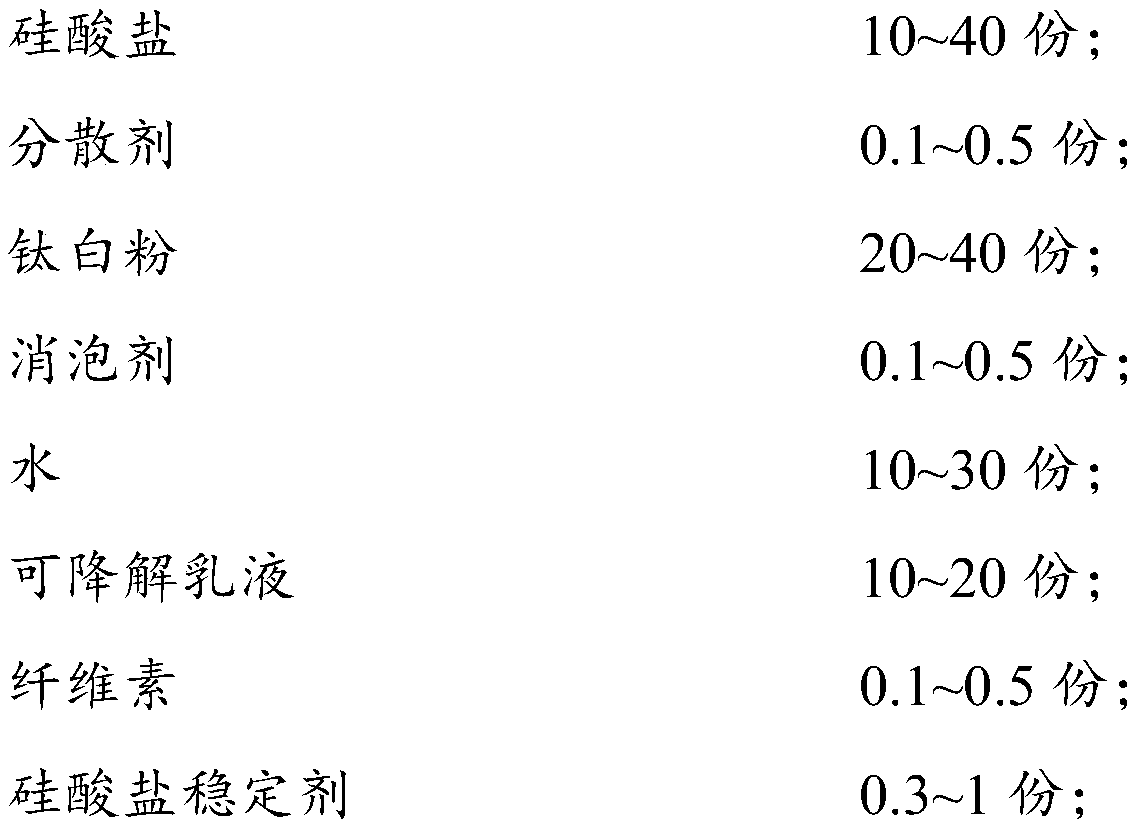
Interior wall coating and preparation method thereof
InactiveCN109722170AAvoidance of residual monomersCan maintain stabilityCoatingsCelluloseAllergic reaction
The invention provides an interior wall coating. The coating comprises the following components in parts by weight: 10-40 parts of a silicate, 0.1-0.5 part of a dispersant, 20-40 parts of titanium dioxide, 0.1-0.5 part of an anti-foaming agent, 10-30 parts of water, 10-20 parts of a degradable emulsion, 0.1-0.5 part of cellulose, and 0.3-1 part of a silicate stabilizer, wherein raw materials of the degradable emulsion comes from fruit extracts of gramineous plants. The interior wall coating adopts the fruit extracts of the gramineous plants as the main preparation raw materials for the degradable emulsion, residual monomers in the emulsion are avoided, performances of a traditional interior wall coating can be reached, and main allergens (preservatives, mould inhibitors, film-forming auxiliary agents, antifreeze agents and residual monomers in the emulsion) are excluded, so that the probability of allergic reactions of skin contactors or respiratory inhalers is reduced. The invention also provides a preparation method of the interior wall coating.
Owner:东莞大宝化工制品有限公司 +1
Traditional Chinese medicine composition with whitening effect and preparation method thereof
ActiveCN108653566AIncreased whitening efficacyImprove the safety of useCosmetic preparationsToilet preparationsPropolisRubus
The invention belongs to the technical field of traditional Chinese medicine, in particular to a traditional Chinese medicine composition with whitening effect and a preparation method thereof. The traditional Chinese medicine composition is prepared from the following fresh raw materials in parts by weight: 100-750 parts of fresh raw radix astragali, 50-350 parts of fresh radix astragali zymophyte, 30-350 parts of raw radix polygoni multiflori, 20-350 parts of diospyros lotus fruits, 30-350 parts of rhizoma polygonati odorati, 30-500 parts of fructus chaenomelis, 30 to 400 parts of fig, 30 to400 parts of rubus idaeus fruits, 30 to 400 parts of herba taraxaci, and 10-300 parts of black propolis. The traditional Chinese medicine composition and the preparation method have the beneficial effects that (1) in comparison with dry traditional Chinese medicine compositions, the clinical efficacy of the extract of the fresh traditional Chinese medicine composition is obviously improved; (2) the diospyros lotus fruits are added to the traditional Chinese medicine composition, and thus the whitening effect is obviously improved.
Owner:赵国林
Tea tree oil throat spray
InactiveCN108524606AFresh tasteReduce stimulationCosmetic preparationsAntimycoticsSide effectMonilinia laxa
The invention discloses a tea tree oil throat spray. Due to side effects of chlorhexidine, people extract and rectify tea tree oil from crassula arborescens (which belongs to the myrtaceae) of Australia to replace the chlorhexidine so as to produce the tea tree oil throat spray. The tea tree oil throat spray contains tea tree oil in a certain proportion. Australian aborigines use tea tree oil leaves to treat wounds for thousands of years. The tea tree oil tastes refreshing, is more excellent in antibacterial property for various inflammations in an oral cavity and can prevent infection and well inhibit candida albicans. Clinically, the stimulation of the tea tree oil is less than that of the chlorhexidine. The tea tree oil cannot discolor teet.
Owner:NINGBO HI TECH ZONE FANMAI TECH CO LTD
Lidocaine hydrochloride compound medicine injection and preparation method thereof
InactiveCN104274434AReduce the incidence of allergic reactionsHydroxy compound active ingredientsAntipyreticNaCl - Sodium chlorideGlycerol
The invention belongs to the technical field of medicinal preparations, and concretely relates to a lidocaine hydrochloride compound medicine injection and a preparation method thereof. The injection comprises 8mg / ml of lidocaine hydrochloride, 1.3mg / ml of menthol, 2.0mg / ml of sodium chloride, 0.05ml / ml of glycerin, 1.33ml / ml of ethanol and 0.001-0.01mg / ml of polyethylene glycol-12-hydroxystearate. The compound injection has the advantages of excellent long-acting anaesthesia effect, stable medicinal preparation, no hemolysis and low allergy.
Owner:CHENGDU LIST PHARMA
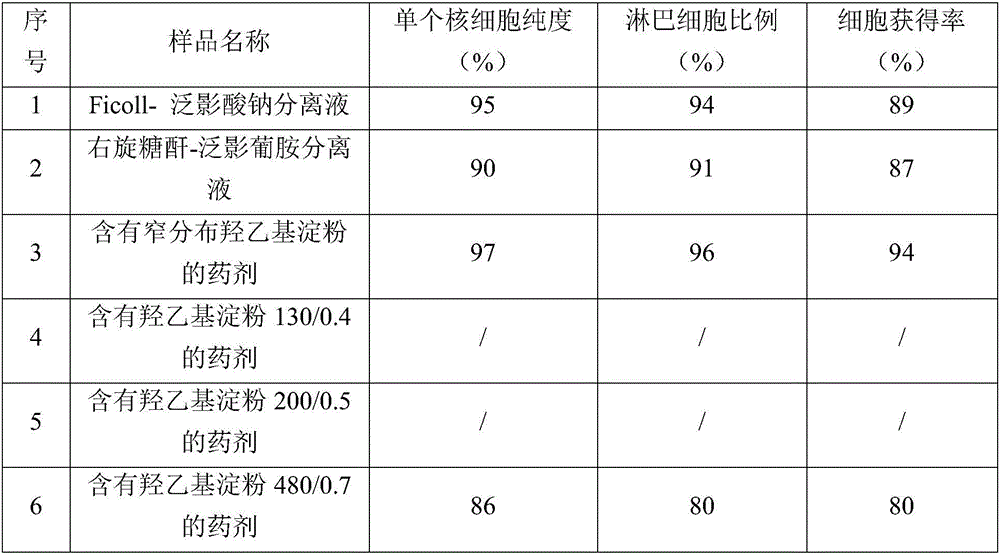
Narrow-distributed hydroxyethyl starch and application thereof
ActiveCN106749710AIncrease vitalityGood conditionCell dissociation methodsArtificial cell constructsTissue densityWeight distribution
The invention discloses narrow-distributed hydroxyethyl starch and application thereof. The weight-average molecular weight Mw of the narrow-distributed hydroxyethyl starch is 250000-400000; the molar substitution degree MS of the narrow-distributed hydroxyethyl starch is 0.3-0.7; the ratio of C2 to C6 is 4-12; the narrow-distributed hydroxyethyl starch can be used as a reagent of a medium for animal tissue or human body tissue density separation, and particularly a medium for blood density separation. By improving key molecular weight distribution of hydroxyethyl starch, such as the weight-average molecular weight Mw, the molar substitution degree MS and the ratio of C2 to C6, compared with the prior art, the narrow-distributed hydroxyethyl starch can be used as a novel medium for animal tissue or human body tissue density separation, so that the problems that a cell separation liquid has toxicity for cells, cell activity can be affected and the like can be effectively solved.
Owner:WUHAN HUST LIFE SCI & TECH +1
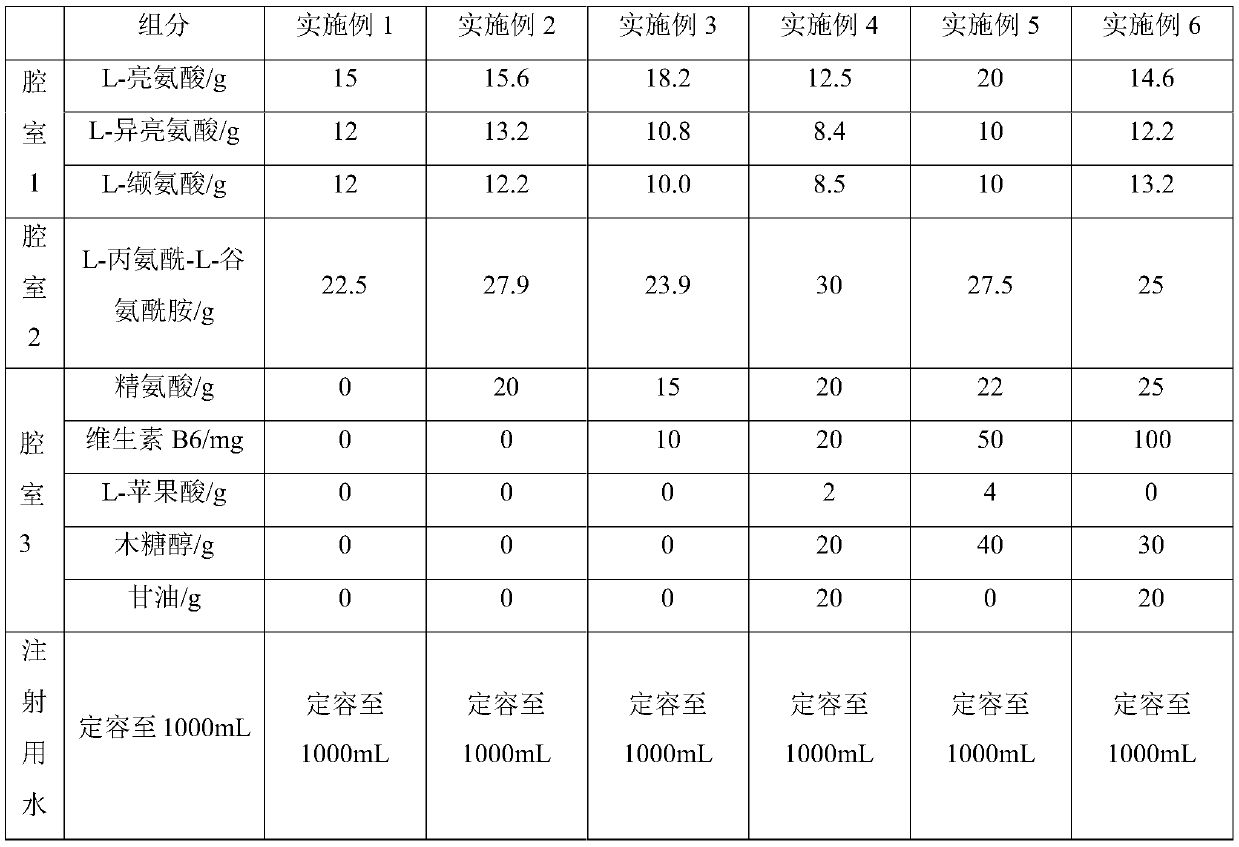
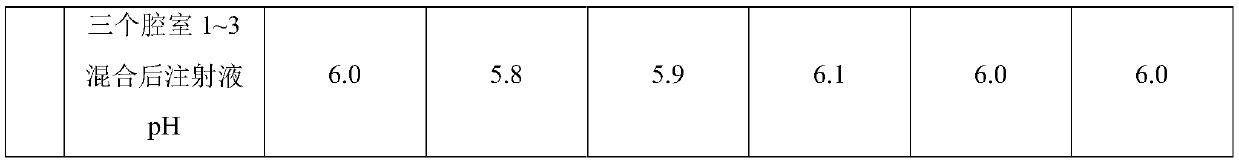
Compound amino acid dipeptide injection as well as preparation method and application thereof
ActiveCN110548129AAvoid decompositionImprove material and energy metabolismDipeptide ingredientsHydroxy compound active ingredientsL-alanyl-l-glutamineDipeptide
The invention belongs to the field of medicines and provides compound amino acid dipeptide injection as well as a preparation method and application thereof. The injection comprises three cavity bags;the three cavity bags adopt three spaced chambers in which a branched-chain amino acid solution, an L-alanyl-L-glutamine solution and a mixed solution containing arginine hydrochloride and vitamin B6are accommodated correspondingly; in each 1000 mL of injection, the total mass of the amino acid is 50 to 130 g; the branched-chain amino acid accounts for 10 to 80 percent of the total mass of the amino acid; the L-alanyl-L-glutamine accounts for 5 to 70 percent of the total mass of the amino acid; the arginine hydrochloride accounts for 0 to 50 percent of the total mass of the amino acid; and the mass of the vitamin B6 is 0 to 1 g. The injection provided by the invention improves product stability and effectiveness while providing product safety; and the technical scheme is simple and convenient and is favorable for industrialized production.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
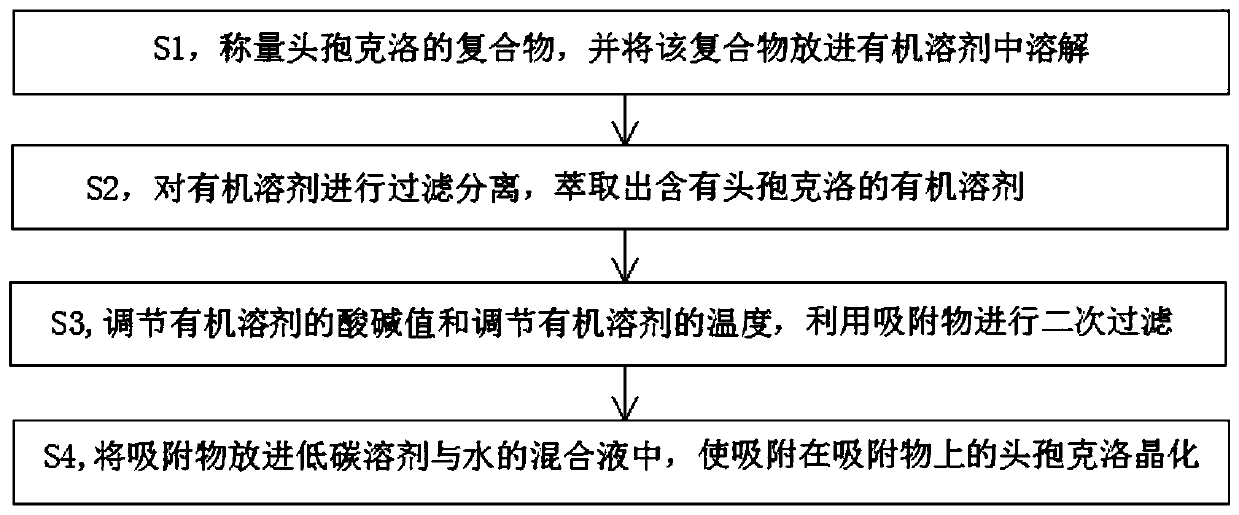
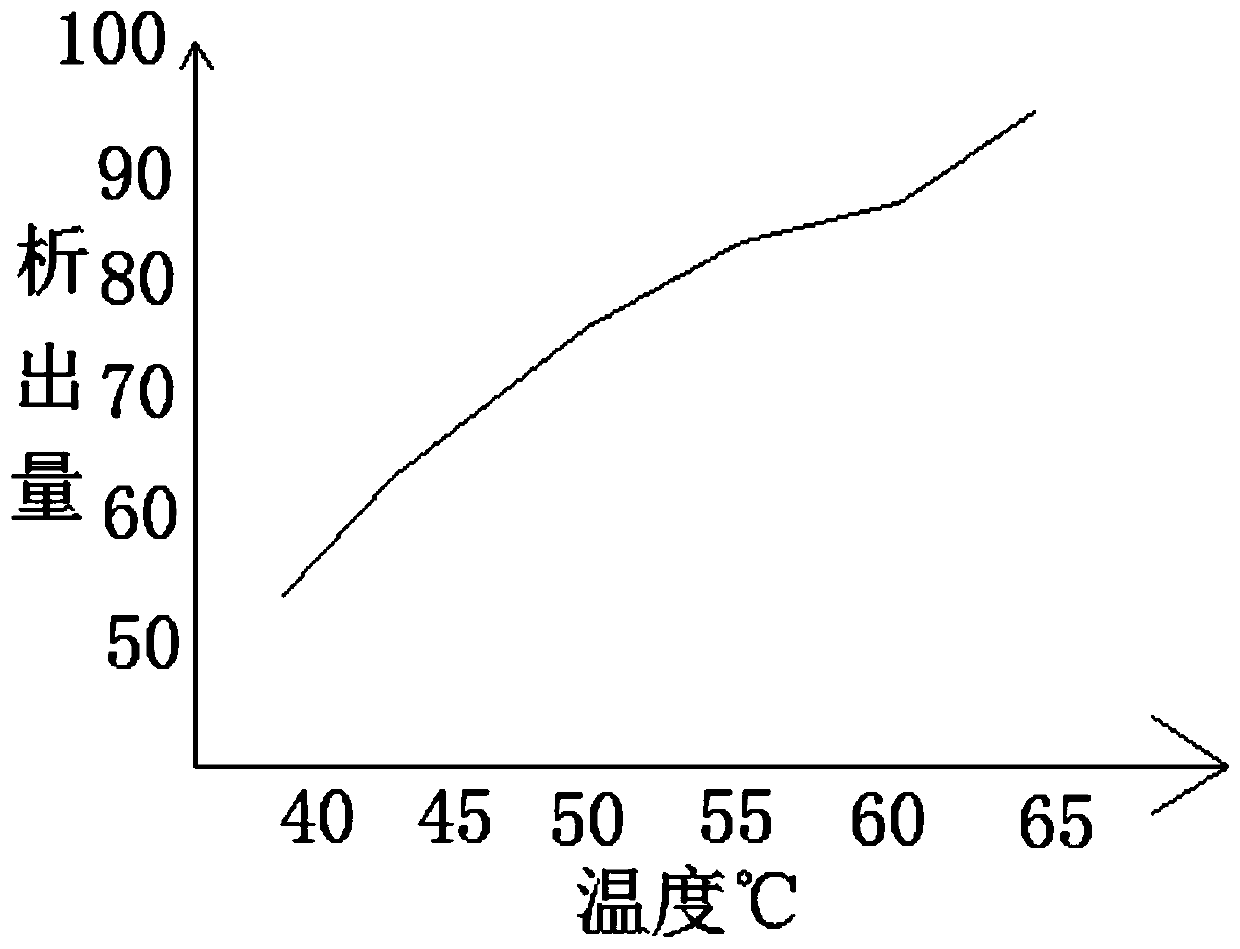
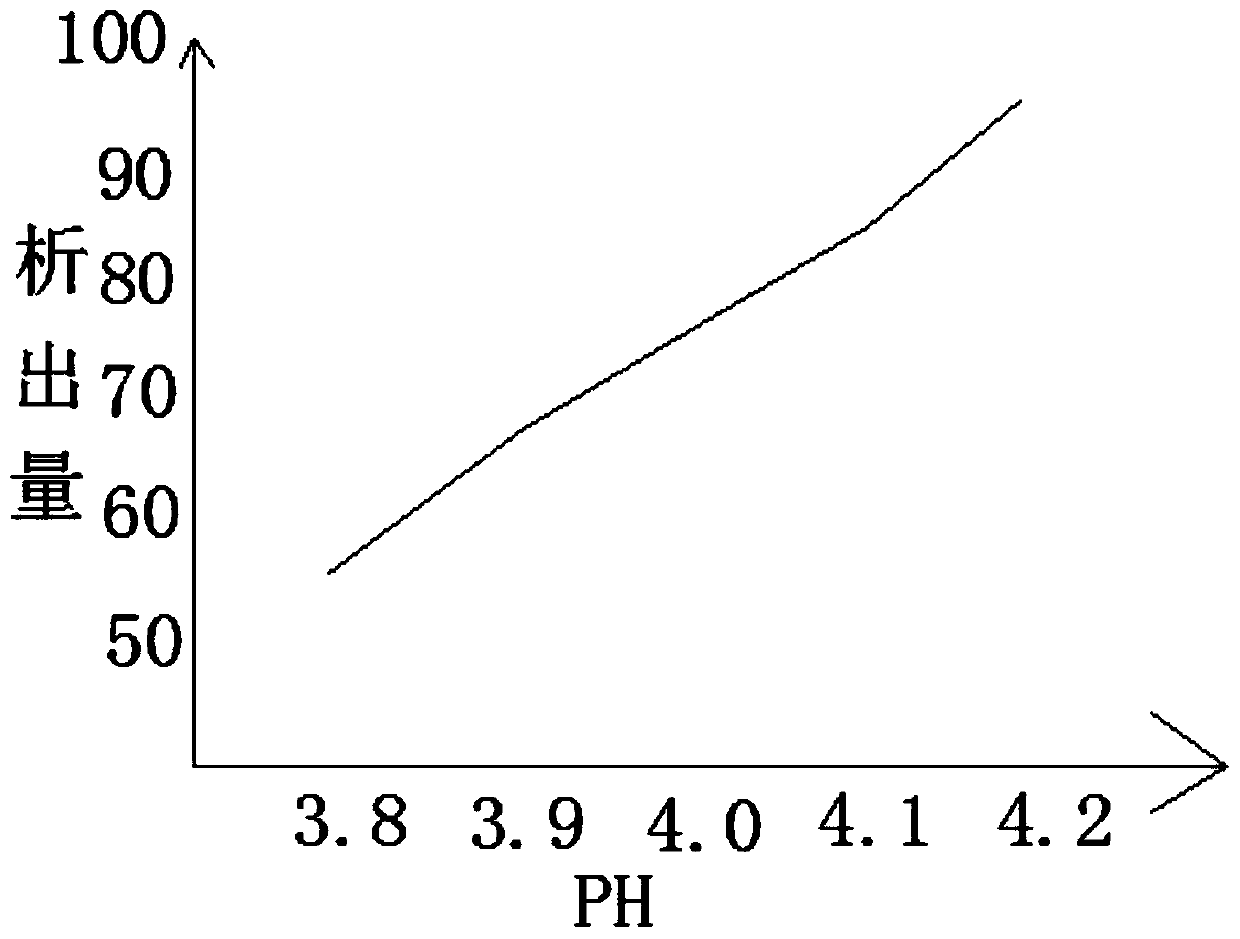
Method for removing cefaclor impurities
InactiveCN111057072AReduce the amount of impuritiesReduce the incidence of allergic reactionsOrganic chemistryOrganic solventPhysical chemistry
The invention relates to the field of medicine purification, and in particular, relates to a cefaclor impurity removal method which comprises the following steps: S1, weighing a cefaclor compound, anddissolving the compound in an organic solvent; S2, filtering and separating the organic solvent, and extracting the organic solvent containing cefaclor; S3, adjusting the acid-base value of the organic solvent and adjusting the temperature of the organic solvent, and performing secondary filtration by utilizing an adsorbate; S4, putting the adsorbate into a mixed solution of a low-carbon solventand water, and crystallizing cefaclor adsorbed on the adsorbate. According to the cefaclor impurity removal method, cefaclor impurities are put in the organic solvent for dissolving, cefaclor insoluble in water can be dissolved in the organic solvent, the organic solvent of cefaclor is separated, filtered and extracted, the pH value is controlled, cefaclor particles with low impurity content are obtained, and the occurrence rate of allergic reaction possibly caused by excessive impurities of medicines is reduced.
Owner:广州维奥康药业科技有限公司
Application of insulin pump into insulin allergy treatment
InactiveCN101966356AReduce the incidence of allergic reactionsAchieve desensitization effectPressure infusionCurative effectInsulin pump
The invention relates to an insulin pump, in particular to application of an insulin pump into insulin allergy treatment. The insulin pump can reduce the incidence rate of an anaphylactic reaction effectively to achieve the curative effect of hyposensitization, and is the ideal selection for patients who depend on the treatment of insulin and are anaphylactic to the insulin. The insulin pump solves the problem of clinical treatment, and simultaneously, provides the new indication for the use.
Owner:HARBIN MEDICAL UNIVERSITY
Method for preparing bone peptide injection
ActiveCN101933944BEfficient removalEnsure safetyMetabolism disorderPharmaceutical delivery mechanismForeign proteinFiltration
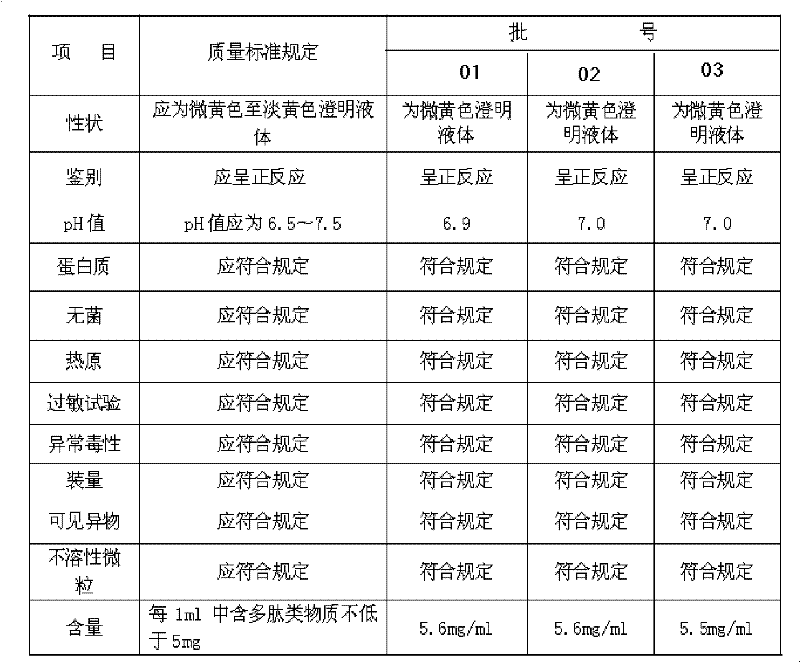
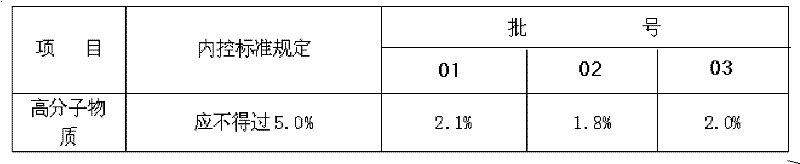
The invention discloses a method for preparing a bone peptide injection. Foreign protein is removed with cold acetone and acid-alkali deposition, and ultra-filtration for multiple stages is carried out through ultra-filtration films of 50, 10 and 5KD; therefore, the method not only can completely remove the foreign protein but also can effectively control high-molecular matter so that the high-molecular matter in the bone peptide injection is less than 2.1% which is greatly lower than state standard, thereby effectively ensuring the safety of preparation.
Owner:武汉华龙生物制药有限公司
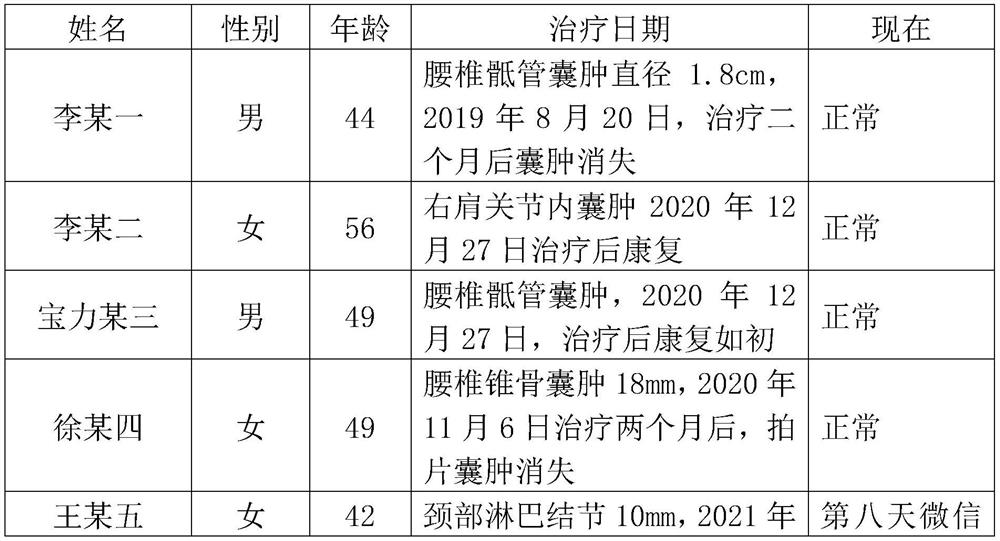
Traditional Chinese medicine for treating sacral canal cyst, lymphatic accumulation and nodule, and preparation method thereof
PendingCN113181225AImprove permeabilityImprove adhesionSkeletal disorderUnknown materialsAllergic reactionChinese herbology
The invention discloses a traditional Chinese medicine for treating sacral canal cyst, lymphatic accumulation and nodule, and a preparation method thereof. The traditional Chinese medicine is prepared from, by weight, 5-6 parts of fibrilia, 5-6 parts of animal oil, 4-15 parts of radix angelicae, 4-15 parts of radix angelicae pubescentis and 6-8 parts of antler. According to the invention, the animal oil is adopted, so that the permeability and adhesive force of the medicine to the skin can be increased, particularly the adhesive animal oil is sticky, the allergic components of a patient in the formula are changed after the medicines are combined, and the traditional Chinese medicine has the characteristics of short treatment time, quick effect, thorough rehabilitation and low allergic reaction rate in the treatment of sacral canal cyst, lymphatic accumulation and nodule.
Owner:乌兰托娅 +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com