Lidocaine hydrochloride compound medicine injection and preparation method thereof
The technology of lidocaine hydrochloride and injection is applied in the field of lidocaine hydrochloride compound drug injection preparation and preparation thereof, and can solve the problems of elevation, easy rancidity, risk of clinical medication and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Prescription 1
[0098] Lidocaine Hydrochloride 8.0g
[0099] Menthol 1.3g
[0101] Glycerin 50.0ml
[0102] Ethanol 133.0ml
[0103] HS15 5.0ml
[0104] Add water for injection to 1000ml
[0105] Makes 200 bottles
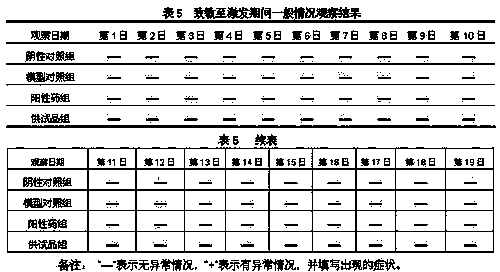
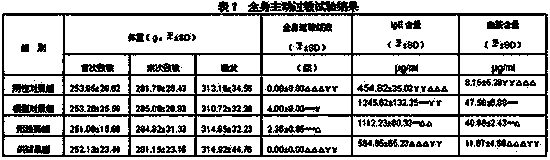
[0106] Take the prescribed amount of menthol, add ethanol to dissolve, wait until the menthol is completely dissolved, then add lidocaine hydrochloride and stir, after completely dissolving, add glycerin, HS15, sodium chloride in sequence, then add water for injection at 35-45°C to mix and adjust To the prescription amount, then adopt multi-stage filtration, filter with a 0.22 μm sterilizing filter at the end, wash and dry the linkage production line to wash the bottle, dry the bottle and fill it, sterilize (F0>8), and pack it.
Embodiment 2
[0107] Example 2 Prescription 2
[0108] Lidocaine Hydrochloride 8.0g
[0109] Menthol 1.3g
[0110] Sodium chloride 2.0g
[0111] Glycerin 50.0ml
[0112] Ethanol 133.0ml
[0113] HS15 1.0ml
[0114] Add water for injection to 1000ml
[0115] Makes 200 bottles
[0116] Take the prescribed amount of menthol, add ethanol to dissolve, wait until the menthol is completely dissolved, then add lidocaine hydrochloride and stir, after completely dissolving, add glycerin, HS15, sodium chloride in sequence, then add water for injection at 35-45°C to mix and adjust To the prescription amount, then adopt multi-stage filtration, filter with a 0.22 μm sterilizing filter at the end, wash and dry the linkage production line to wash the bottle, dry the bottle and fill it, sterilize (F0>8), and pack it.
Embodiment 3
[0117] Example 3 Prescription 3
[0118] Lidocaine Hydrochloride 8.0g
[0119] Menthol 1.3g
[0120] Sodium chloride 2.0g
[0121] Glycerin 50.0ml
[0122] Ethanol 133.0ml
[0123] HS15 10.0ml
[0124] Add water for injection to 1000ml
[0125] Makes 200 bottles
[0126]Take the prescribed amount of menthol, add ethanol to dissolve, wait until the menthol is completely dissolved, then add lidocaine hydrochloride and stir, after completely dissolving, add glycerin, HS15, sodium chloride in sequence, then add water for injection at 35-45°C to mix and adjust To the prescription amount, then adopt multi-stage filtration, filter with a 0.22 μm sterilizing filter at the end, wash and dry the linkage production line to wash the bottle, dry the bottle and fill it, sterilize (F0>8), and pack it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com