Veal-blood protein-removed extract infusion and its preparation process
A preparation process, calf blood technology, applied in the directions of drug delivery, peptide/protein composition, drug combination, etc., can solve the problems of increasing the workload of medical staff, increasing the incidence of adverse reactions, increasing clinical allergic reactions, etc., and achieving clinical drug use Safety, reducing the incidence of allergic reactions, and promoting the effect of intake and utilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
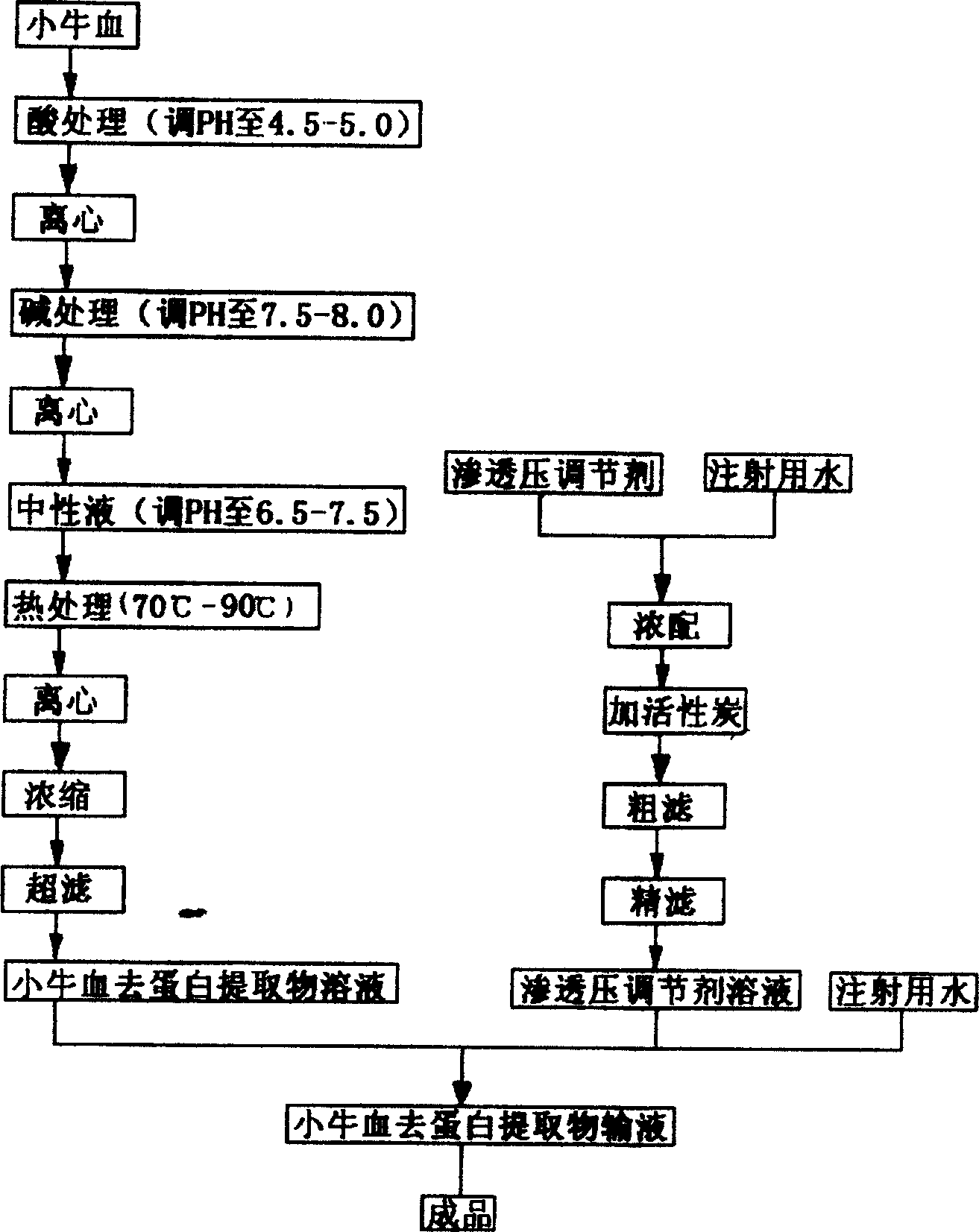
[0018] Calf blood deproteinized extract infusion and its preparation process, its composition includes: calf blood deproteinized extract and osmotic pressure regulator, the calf blood deproteinized extract is extracted from calf blood or serum , the calf blood deproteinized extract solid contains total nitrogen and amino acids, its parts by weight are amino acid 20.0, total nitrogen 12.5, osmotic pressure regulator 50, pH value should be 5.5, this product is light yellow or light yellow Yellow clear liquid. The preparation process is as follows: take 16,500 parts by weight of calf blood, filter to obtain a crude filtrate with 16,000 parts by weight, adjust the pH to 4.5, and centrifuge to obtain a supernatant with 15,000 parts by weight, and adjust the pH value to 7.5, centrifuged to obtain a supernatant of 12,000 parts by weight, take the supernatant, adjust the pH value to 6.5, heat-treat at 70°C, centrifuge, concentrate under reduced pressure at 60°C to obtain a concentrate...
Embodiment 2
[0020] Calf blood deproteinized extract infusion and its preparation process, its composition includes: calf blood deproteinized extract and osmotic pressure regulator, the calf blood deproteinized extract is extracted from calf blood or serum , the calf blood deproteinized extract solid contains total nitrogen and amino acids, its parts by weight are amino acid 30.0, total nitrogen 18.75, osmotic pressure regulator 100, pH value should be 8.0, this product is light yellow or light yellow Yellow clear liquid. The preparation process is as follows: take 24,800 parts by weight of calf blood, filter to obtain a crude filtrate with 24,000 parts by weight, adjust the pH value to 5.0, and centrifuge to obtain a supernatant liquid with 23,000 parts by weight, and adjust the pH value to 8.0, centrifuged to obtain a supernatant of 20,000 parts by weight, take the supernatant, adjust the pH value to 7.5, heat-treat at 90°C, centrifuge, concentrate under reduced pressure at 80°C to obtai...
Embodiment 3
[0022] Calf blood deproteinized extract infusion and its preparation process, its composition includes: calf blood deproteinized extract and osmotic pressure regulator, the calf blood deproteinized extract is extracted from calf blood or serum , the calf blood deproteinized extract solid contains total nitrogen and amino acids, its parts by weight are amino acid 25.0, total nitrogen 15.5, osmotic pressure regulator 70, pH value should be 6.5, and this product is light yellow or light Yellow clear liquid. The preparation process is as follows: take 22,800 parts by weight of calf blood, filter to obtain a crude filtrate with 22,000 parts by weight, adjust the pH value to 4.7, and centrifuge to obtain a supernatant liquid with 22,000 parts by weight, and adjust the pH value to 7.8, centrifuged to obtain a supernatant of 15,000 parts by weight, take the supernatant, adjust the pH value to 7.0, heat-treat at 80°C, centrifuge, concentrate under reduced pressure at 70°C to obtain a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com