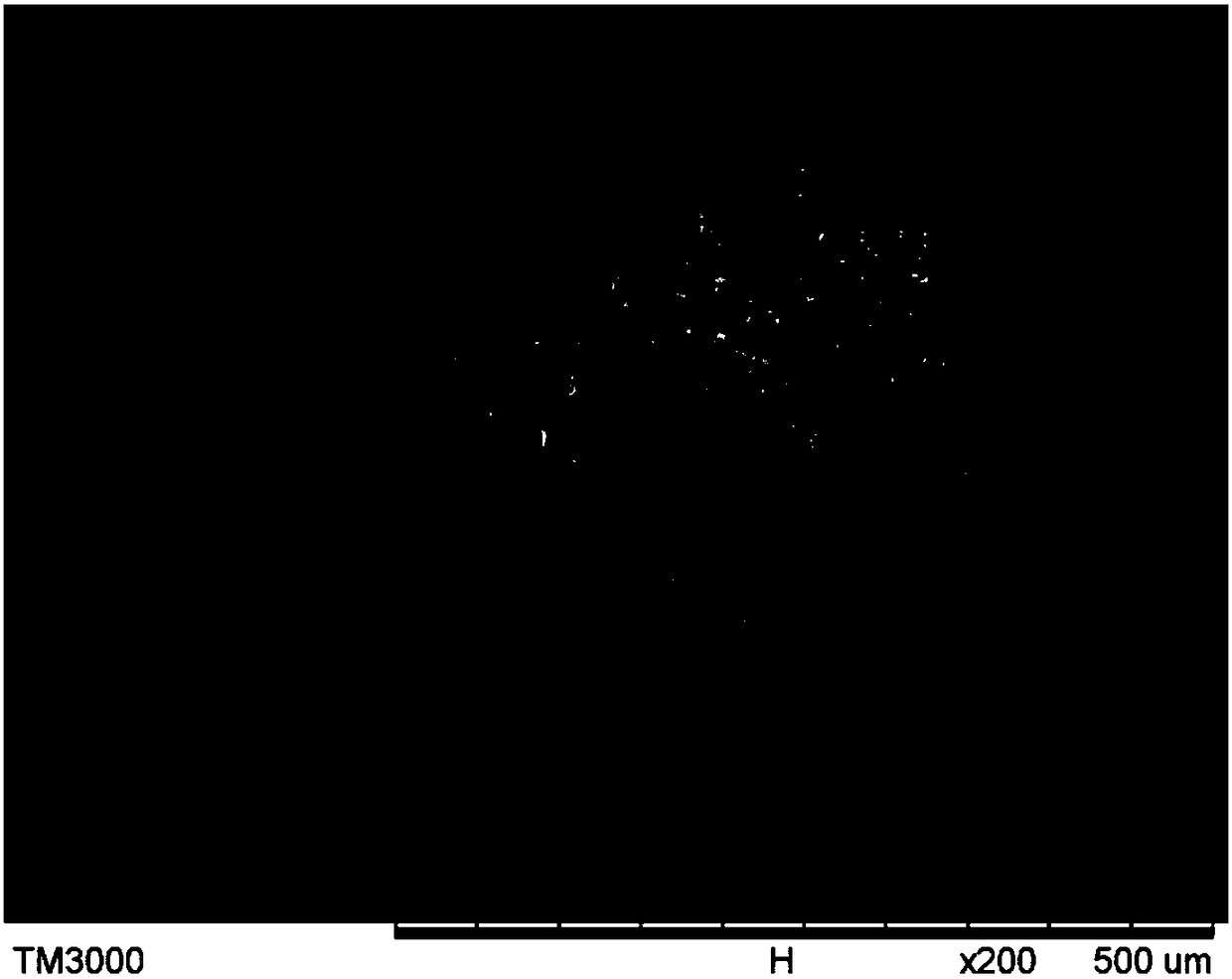
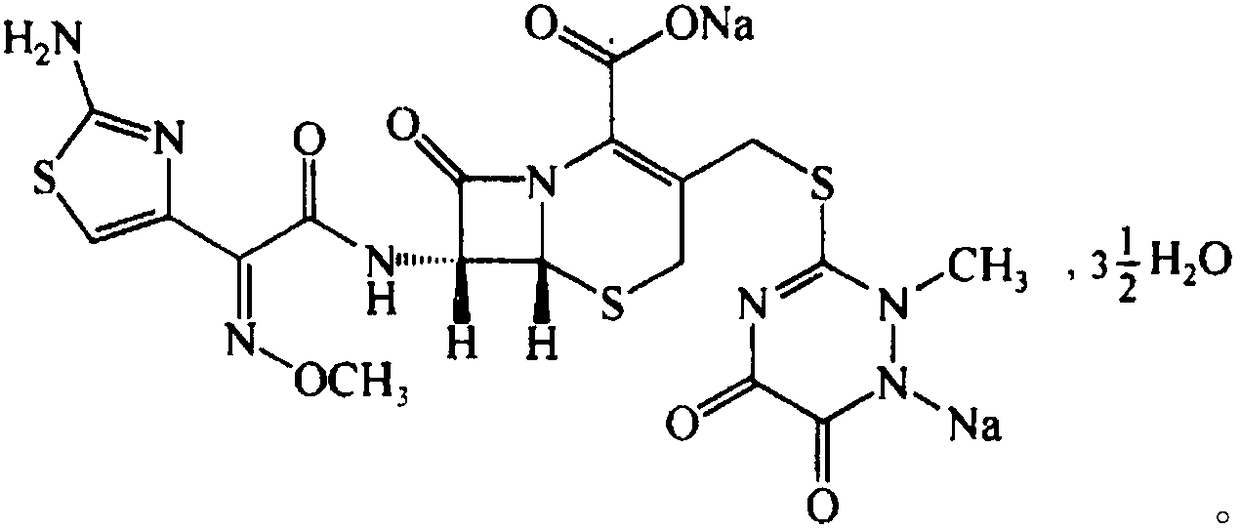
Preparation method of ceftriaxone sodium spherical crystals
A technology for ceftriaxone sodium and spherical crystals is applied in the field of preparation of ceftriaxone sodium spherical crystals, which can solve the problems of small particle size and poor fluidity of ceftriaxone sodium, and achieve good fluidity, improved physical properties, and single-pass yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] The embodiment of the present invention provides a kind of preparation method of ceftriaxone sodium spherical crystal, comprises the following steps:
[0020] S01: Provide ceftriaxone sodium API, bridging agent and dissolution agent;
[0021] S02: dissolving the ceftriaxone sodium bulk drug in a polar solvent to obtain a first ceftriaxone sodium solution;
[0022] S03: mixing the bridging agent with the first ceftriaxone sodium solution to obtain a second ceftriaxone sodium solution;
[0023] S04: mixing the dissolving agent with the second ceftriaxone sodium solution to precipitate spherical ceftriaxone sodium crystals.
[0024] The preparation method of spherical crystals of ceftriaxone sodium provided by the embodiments of the present invention comprises dissolving the bulk drug of ceftriaxone sodium in a polar solvent and adding a suitable bridging agent. The liquid bridging agent coalesces and directly converts the flake crystals produced by elution crystallizati...
Embodiment 1
[0036] Get 22g ceftriaxone sodium crude drug and be dissolved in 44g distilled water, add the saturated sodium chloride solution of 2g again, continue to stir for 10 minutes, filter mixed solution, remove undissolved solid, filtrate is transferred in the crystallizer, and use Wash the filter flask with 3-5ml of distilled water. Continue to add 54 g of methyl acetate into the crystallization mother liquor, mix and stir for 20 minutes, and control the temperature of the system at 15°C. After mixing completely, start to add the dissolution agent acetone dropwise, the dosage of the dissolution agent is 8 times of the raw material drug, and the dissolution agent is controlled to be added dropwise within 4 hours, and the stirring rate is controlled to be 200r / min in this process. After the dissolution agent is added dropwise, keep stirring and continue crystal growth for 1.5 hours. After the crystal growth is completed, filter and wash the filter cake with acetone solvent, and dry ...
Embodiment 2
[0038] Get 22g ceftriaxone sodium crude drug and be dissolved in 44g distilled water, add the saturated potassium chloride solution of 2g again, stir continuously 10 minutes, filter mixed solution, remove undissolved solid, filtrate is transferred in the crystallizer, and use Wash the filter flask with 3-5ml of distilled water. Continue to add 40 g of ethyl acetate solvent into the crystallization mother liquor, mix and stir for 20 minutes, and control the system temperature at 15°C. After mixing completely, start to drop dissolving agent acetone, the consumption of dissolving agent is 8 times of raw material drug, control dissolving agent dropwise within 5 hours, this process control stirring speed is 200r / min. After the dissolution agent was added dropwise, keep stirring and continue growing the crystal for 2 hours. After the crystal growth is completed, filter and wash the filter cake with acetone solvent, and dry the obtained product in a vacuum oven at 30°C for 5 hours. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com