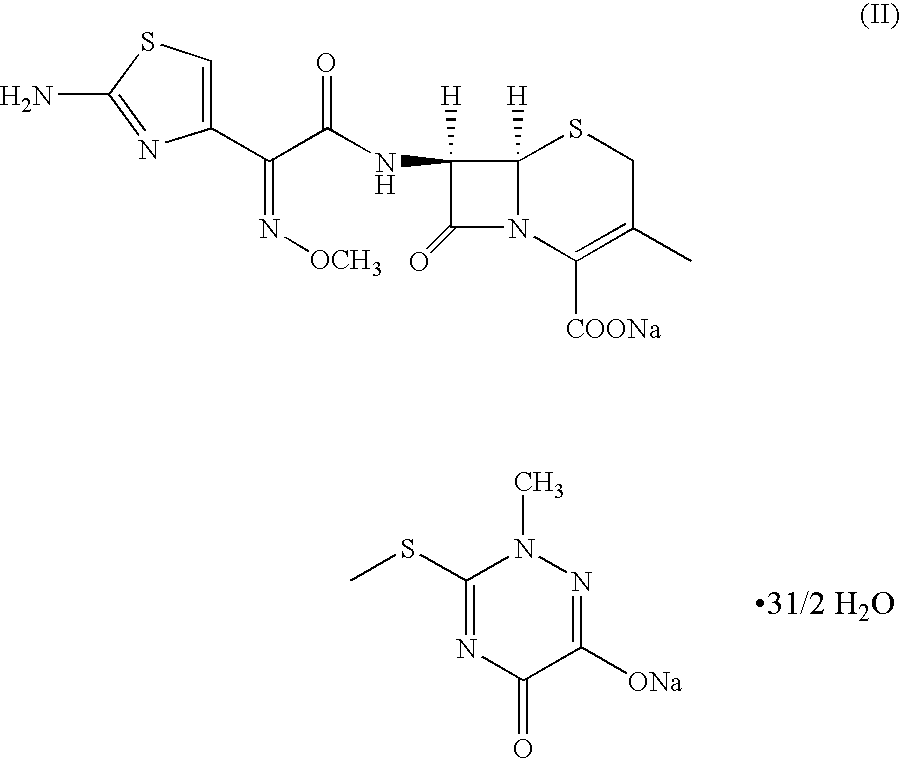
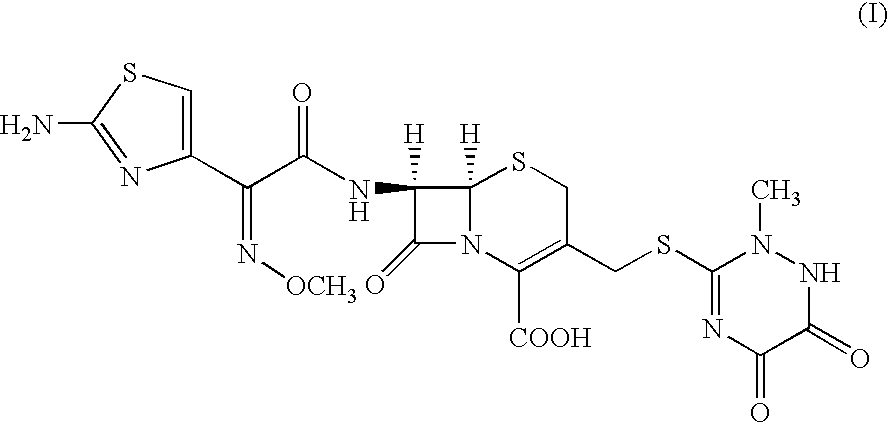
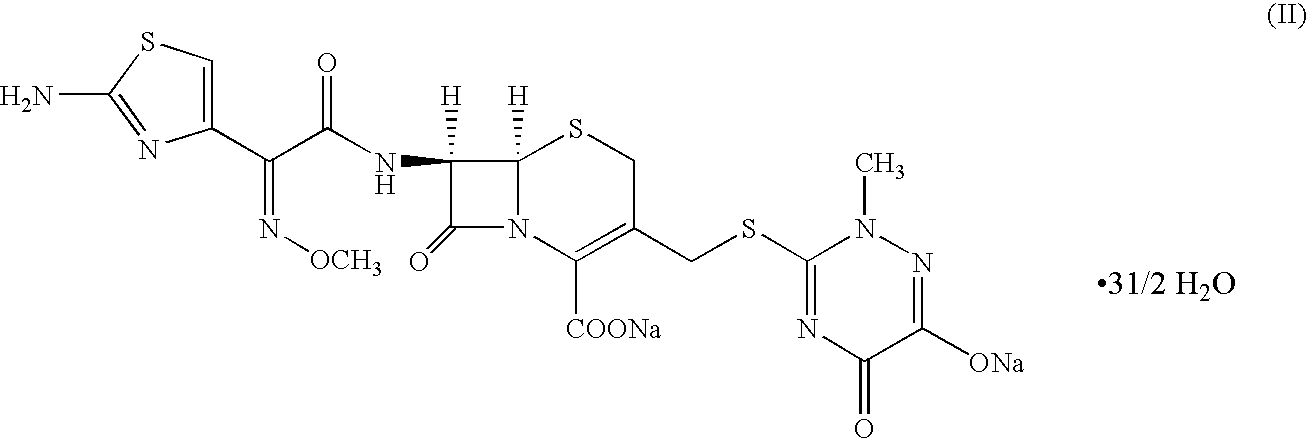
Method for manufacture of ceftriaxone sodium
a technology of ceftriaxone and sodium ceftriaxone, which is applied in the field of improved methods for manufacturing of ceftriaxone sodium, can solve the problems of inconsequential changes in parameters and solvents, no bearing on the course of reaction, and increase the overall cost of coupling reaction, etc., and achieves high yield and purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example — 1
REFERENCE EXAMPLE—1
Preparation of ceftriaxone sodium (II) as Per the Method Described in Example—2 of U.S. Pat. No. 6,552,186 B2
Step—1: Preparation of (N,O)-bis trialkylsilyl 7-amino-3-[2,5-dihydro-6-hydroxy-2-methyl5-oxo-as-triazin-3-yl]-3-cephem-4-carboxylic acid (III)
50 gm (0.135 moles) of 7-amino-3-{[(2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3-yl)thio]methyl)}-3-cephem-4-carboxylic acid was suspended in dichloromethane (500 ml). An additional amount of 1000 ml of dichloromethane was added to the suspension and distilled out to effect azeotropic removal of water. To the suspension was added Bis silyl acetamide (109.70 gm; 0.540 moles) at 25° C. to 30° C. and the mixture agitated under an atmosphere of nitrogen for 2 hours. The solution of (N,O)-bis trialkylsilyl 7-amino-3-[2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3-yl]-3-cephem-4-carboxylic acid (III) thus obtained was cooled to −10° C.
Step—2: Preparation of 4-bromo-2-methoxyimino-3-oxo Yutyric acid chloride (IV...
example — 2
EXAMPLE—2
Preparation of ceftriaxone sodium (II) as Per the Method of the Present Invention Utilizing 4-halo-2-methoxyimino-3-oxo butyric acid (IV) having a Purity of 87% in the Absence of an Acid Scavenger
Step—1: Preparation of (N,O)-bis trialkylsilyl 7-amino-3-[2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3 yl]-3-cephem-4-carboxylic acid (III)
A suspension of 7-amino-3-{[(2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3yl)thio]methyl)}-3-cephem-4-carboxylic acid (100 gm; 0.270 moles) and dichloromethane (2700 ml) was heated to reflux and 2000 ml of dichloromethane was distilled out till moisture content of the reaction mixture is below 0.06%. The reaction mixture was cooled to room temperature. To this was added 74.0 gm (0.458 moles) of hexamethyldisilazane and trimethylchlorosilane (10.8 gm; 0.0095 moles) and the mixture refluxed for 8 hours. The solution containing (N,O)-bis trialkylsilyl 7-amino-3-[2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3-yl]-3-cephem-4-carboxylic...
example — 3
EXAMPLE—3
Preparation of ceftriaxone sodium (II) as Per the Method of the Present Invention Utilizing 4-halo-2-methoxyimino-3-oxo butyric acid (IV) having a Purity of 97% and Prepared as the Method Disclosed in WO03 / 045899 in the Absence of an Acid Scavenger
Step—1: Preparation of (N,O)-bis trialkylsilyl 7-amino-3-[2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3 yl]-3-cephem-4-carboxylic acid (III)
A suspension of 7-amino-3-{[(2,5-dihydro-6-hydroxy-2-methyl-5-oxo-as-triazin-3-yl)thio]methyl)}-3-cephem-4-carboxylic acid (100 gm; 0.270 moles) and dichloromethane (2700 ml) was heated to reflux and 2000 ml of dichloromethane was distilled out till moisture content of the reaction mixture is below 0.06%. The reaction mixture was cooled to room temperature. To this was added 74.0 gm (0.458 moles) of hexamethyldisilazane and trimethylchlorosilane (10.8 gm; 0.0095 moles) and the mixture refluxed for 8 hours. The solution containing (N,O)-bis trialkylsilyl 7-amino-3-[2,5-dihydro-6-hydroxy-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com